
How to perform periodic review of systems and processes in pharmaceuticals?
- Published on: Nov 10, 2021
Imagine you have a new facility where all laboratory instruments are qualified, and all computer systems controlling the production facility are validated. Packaging lines are fully automated and working without any hiccups.
Aseptic facilities are free from any microbial particulates. No issue with product sterilization. Your testing methods are so robust to generate any error or cleaning methods are so rigorously validated that you are not worried about cross-contamination. The quality and safety of your products are guaranteed!
Doesn’t it sound like an ideal world? Sounds too good to be true.
In fact, from time to time you should expect to have machine breakdowns, setbacks, power surges, software glitches, human errors, unplanned deviations and poorly managed changes due to aging of equipment, change in environment, wear and tear, etc.
If those variations and trends are not identified and managed proactively, all of your systems and processes will eventually start to collapse. The quality and safety of your product will suffer immensely.
To avoid such detrimental situations, pharmaceutical businesses employ proven strategies such as risk-based periodic reviews of systems and processes that have high impacts on product quality.
What is periodic review of validated systems and processes?
USFDA – Guidance for Industry Q7A, Chapter XII, section F (periodic Review of Validated Systems) quotes “Systems and processes should be periodically evaluated to verify that they are still operating in a valid manner”.
In order to make sure that drugs are manufactured safely and effectively, it’s really important that you have a plan in place for periodic review of validated systems and processes used to manufacture those medicinal products.
This periodic review plan should include regular checks of systems and processes that come in contact with the drug materials during production.
It’s also important to periodically evaluate both the validated and qualified equipment and computer systems to ensure that they’re still in good shape.
If there are any high-risk processes and equipment, such as processes used in sterile or aseptic production, then it might be necessary to do routine re-qualification and/or revalidation.
By taking a risk-based approach, you can prioritize which equipment needs to be reviewed and how often. You can generate scientific justification for how often do you need to review and re-qualify/revalidate the equipment.
What is the primary reason for periodic review of validated processes?
Periodic reviews are used as an early warning mechanism to ensure that the validated systems and processes that are directly used to prepare medicinal products remain effective, efficient, consistent, and safe over their lifetime.
Even though a process may have been validated initially, changes in equipment, environment, personnel, or raw materials could affect the process’s performance.
Periodic reviews help us to identify any such changes and ensure that the process remains in a validated state.
The periodic review process helps to ensure that the manufacturing process continues to produce high-quality products, remains compliant with regulatory expectations, and reduces the risk of errors or safety incidents.
By regularly reviewing our systems and processes and making any necessary changes, we can maintain the highest level of quality and safety in our products.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Periodic review vs routine revalidation of systems and processes?
There is a significant difference in understanding between periodic review and revalidation.
A periodic review involves regularly reviewing the systems, processes, and procedures used in the pharmaceutical industry to ensure that they remain in their validated state over time.
The purpose of the periodic review is to identify any changes that may have occurred since the last review.
The outcome of the periodic review process determines if a revalidation of low-risk process is necessary and when.
Revalidation, on the other hand, is a process of performing validation activities on previously validated systems or processes.
The purpose of revalidation is to verify that the equipment, facilities, and processes used in pharmaceutical industry continue to meet the required specifications, comply with regulatory requirements, and produce products of consistent quality.
Routine revalidation is typically required for high-risk processes such as sterile manufacturing, aseptic processing, and critical equipment cleaning. However, it may also be required for other processes that could affect product quality or safety.
When to conduct routine process revalidation or requalification?
Routine revalidation is only required for high-risk processes (e.g. sterile/aseptic).
The types of processes that are typically involved in routine revalidation include sterilization and aseptic processing (e.g. autoclaves, de-pyrogenation tunnels, steam-in-place systems, aseptic filling Lines, etc).
If routine revalidation is required, it is typically conducted using a concurrent validation approach.
The testing is executed on a predetermined frequency against a standardized pre-approved protocol or standard operating procedure.
If we need to make changes to a process or a validated system during the routine revalidation process (for example, changing the loading pattern in an autoclave), we need to follow a two-step revalidation process.
First, we should requalify the previously validated parameters to demonstrate that the process has remained under a state of control since the last re-qualification.
After that, we can make the necessary changes and then validate the process prospectively.
When conducting the routine revalidation process, we need to perform at least one validation run and define the details of the required tests (for example, load configuration and container size) in the routine revalidation procedure.
This helps us ensure that the process remains effective and safe while still allowing for necessary changes to be made.
Who is responsible for completing the periodic review?
In pharmaceutical industry, completing the periodic review is a shared responsibility between different departments and personnel.
The exact responsibilities may vary depending on the size of the organization, but typically the Validation / Technical Services, Engineering, and Quality Assurance (QA) departments take a lead role in managing and coordinating the periodic review process.
i. Validation / Technical Services
– Maintain status and schedule of performed and upcoming periodic reviews.
– Perform risk assessment for systems and processes.
– Complete the periodic review when required.
– Review and approve the periodic review report.
ii. Engineering & Production
– Perform risk assessment for systems and processes.
– Perform and complete periodic reviews when required.
iii. Quality Assurance
– To review and approve all periodic review report(s).
– Perform risk assessment for systems and processes.
– Perform and complete periodic reviews when required.
Try our FREE online GMP Skill Booster tests. It’s challanging, it’s refreshing and it’s FREE. Try now!
What are some rules you should follow during the periodic review?
You should follow some basic rules before deciding which validated systems and processes would require periodic reviews as opposed to those that require routine revalidation. For example,
1. Validated systems and processes should be periodically evaluated through periodic review to verify that they remain in a validated state.
2. The periodic review frequency shall be based on the results of risk assessment and/or regulatory requirements.
3. A risk-based approach can be applied to prioritize periodic review and routine re-qualification of validated systems and processes.
4. You should complete an impact analysis to determine whether the system is a direct impact, indirect or non-impact system prior to periodic review.
5. You should include all direct impact systems under the periodic review plan. Direct impact systems mean those systems that directly come in contact with medicinal products.
6. Some examples of direct impact systems are:
– Facilities
– Utilities
– Laboratory test methods,
– Production equipment,
– Computer or automation systems,
– Manufacturing processes and control systems
– Sterile processes
– Equipment cleaning procedures.
7. You should conduct periodic reviews within a time interval not to exceed 5 years.
8. In the absence of a risk-based schedule, you should perform periodic reviews within 2 years by default.
9. Periodic review of the validated processes is not required as the evaluation of deviations, changes and process trends is covered and combined with the annual product records review.
10. The results of the periodic review must be documented, reviewed, and approved by the site validation committee.
11. The review may result in the need for additional studies (e.g. supplemental validation or revalidation).
12. For systems and processes with lower risk, a periodic review with evidence that those systems and processes are consistently producing products that meet their specifications fulfills the need for revalidation.
How often is a periodic review of systems should be performed?
When it comes to direct impact systems in pharmaceuticals, the frequency of periodic reviews is determined based on the level of risk associated with each system.
You should have a process in place for determining the frequency and scheduling plan of these reviews. You should take a procedural approach to document the outcome of the review.
Typically, the validation committee is responsible for approving the assignment of frequencies based on risk, which is determined by looking at the system requirements documentation and any previous impact assessments.
To make sure you get the risk assessment right, representatives from Quality Assurance, system owners, and other stakeholders like Engineering, all should come together to conduct the assessment jointly.
You should evaluate the system’s criticality as a minimum requirement. You can also look at the probability of an adverse event and how easily you can detect it.
These factors will help you justify the level of quality risk associated with a system. These factors play an important role in determining the frequency of periodic reviews.
For example:
i. Low-Risk Priority:
Periodic review frequency of maximum of 5 years. It may also be possible to justify no fixed frequency and use trending of existing systems such as change management, calibration, and preventative maintenance, system security reviews, and system deviations and failures.
The trigger to imitate a periodic review in this case should be clearly defined for each of the supporting systems.
ii. Medium Risk Priority
A periodic review should be conducted at least every 3 years. Actual frequency should be assigned and justified based on system usage.
iii. High-Risk Priority
An annual periodic review is recommended.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
How often is a periodic review of processes should be performed?
To ensure the periodic review process is effective, it is essential to clearly define the scope and initiation of a periodic review for each process, which should be based on a risk assessment.
In this regard, various considerations must be included, and some of them are listed below:
i. Criticality of the process and associated product(s) – e.g. final product, intermediate, bulk, route of administration.
ii. Critical quality attributes of the product that are controlled and/or monitored by the system or process without downstream verification.
iii. Probability of a critical parameter deviation being detected before it could reasonably affect product quality attributes.
iv. Use of data from the system to demonstrate compliance with a registered process, including batch records, lot release, or other GMP records.
v. Ability to trend the systems being used to detect drift that may impact the validation status of the process.
vi. Frequency of trend data recording and review
Periodic review examples of pharmaceutical systems?
During the periodic review of systems, you should evaluate the following steps:
i. Review of system description, including a complete listing of critical subsystems/components (e.g. equipment, hardware, software).
ii. Review of the cumulative and/or repetitive effect of all changes (e.g. Change Controls) to include an assessment of whether further action is warranted.
iii. Review of all deviations (e.g. Deviations, Incidents) including frequency and reasons, to determine whether there is a trend away from the qualified state.
iv. Review of appropriate maintenance and calibration records (as applicable) to determine whether the system has been properly maintained.
v. Review performance trending, if applicable (e.g. system logs).
vi. Review system against applicable regulatory, GMP and Site requirements established since the last periodic review or qualification.
Periodic review examples of pharmaceutical processes?
Below are periodic review considerations for some specific types of processes:
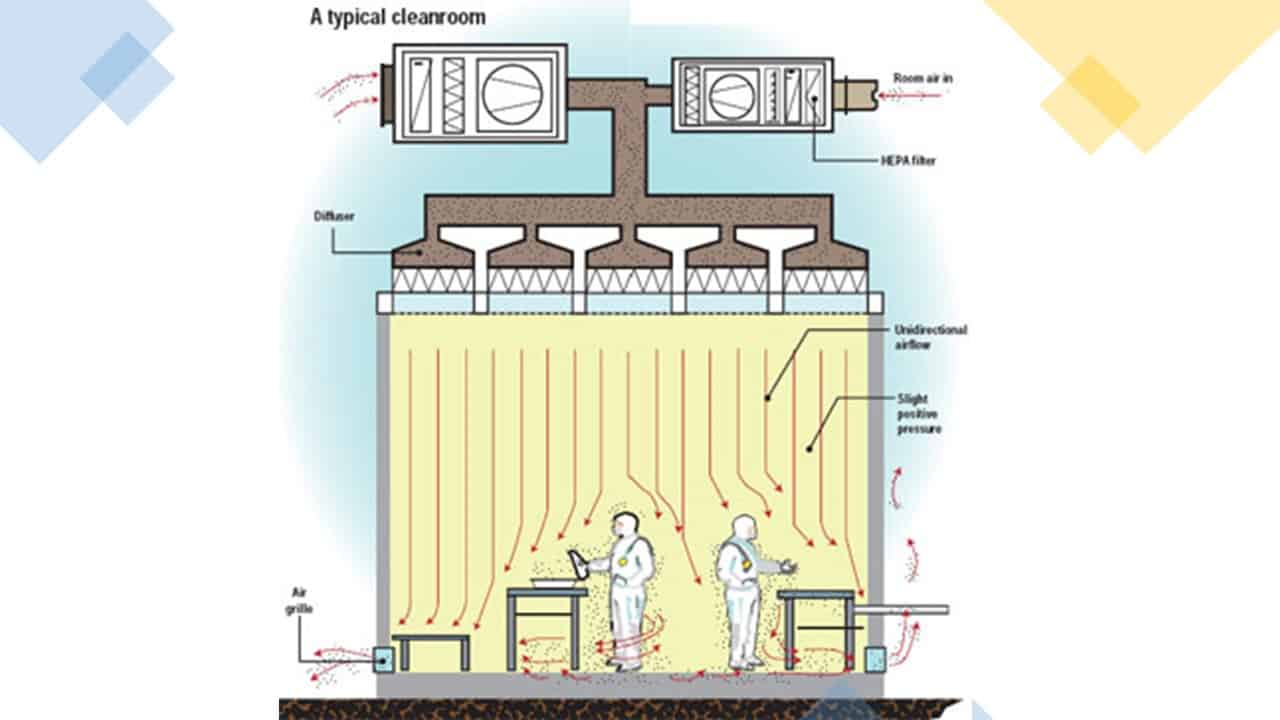
i. Sterile and sterilization processes:
For sterile and sterilization processes that are subject to routine re-qualification due to their criticality, periodic review is not required. The evaluation of deviations, changes, and process trends is covered by the annual product quality review (PRQ).
ii. Manufacturing processes:
You should perform a product quality review for all critical production steps associated with a GMP (Good Manufacturing Practice) site for active pharmaceutical ingredients (API) and marketed drug products.
Depending on the regulatory requirements, the annual PQR may include a validation summary. This meets the need for a periodic review of the process.
iii. Test methods:
Quality control test methods typically have built-in controls such as system suitability tests or trending of assay standards that provide a high level of confidence in the ongoing validity of the method.
In addition, a review of laboratory data, investigations, deviations and associated corrective actions is required in the annual product quality review. This meets the need for periodic review.
What to conclude in a periodic review of systems and processes?
After you perform a periodic review of systems and processes, a report should be written to address the following points as part of the conclusion:
i. The report should state whether the validation documentation reflects the current process or system validation status and make any recommendations for revalidation/re-qualification, and potential continuous improvement of the process.
ii. A confirmation that corrective and preventive actions (CAPA) listed in the document are tracked.
iii. An assessment of the periodic review frequency based on the results obtained and provide a recommendation as to whether it remains appropriate (e.g. will the review frequency be kept the same, reduced, or increased).
The periodic review report should be reviewed and approved by the validation committee, quality assurance, and system owner. It may be archived together with the validation files for the system or process.
Summary
In this post, we have emphasized the importance of performing periodic reviews of validated systems and processes in a pharmaceutical manufacturing environment.
Periodic reviews are critical to ensure that previously validated systems and processes remain in a state of control and consistently produce results that meet predefined requirements.
Some key takeaway points are:
1. Performing periodic reviews of systems and processes is important to ensure pharmaceutical products are safe and effective over their lifetime.
2. Periodic reviews involve evaluating the systems, processes, and procedures within a defined period to ensure that they remain in their validated state since the last review performed.
3. The frequency of periodic reviews for direct impact systems in pharmaceuticals is determined based on the level of risk associated with each system.
4. You should take a risk-based approach while scheduling periodic reviews.
5. You should include all direct impact systems, and conduct periodic reviews within a time interval not to exceed 5 years for low-risk systems and 3 years for medium risks systems.
6. Routine revalidation is typically required for high-risk processes such as sterile manufacturing, aseptic processing, and critical equipment cleaning.
7. Completing the periodic review is a shared responsibility between different departments and personnel such as Validation / Technical Services, Engineering, and Quality Assurance (QA).
8. Systems and processes that should be included in periodic reviews are:
– Facilities
– Utilities
– Laboratory test methods
– Production equipment
– Computer or automation systems
– Manufacturing processes and control systems
– Sterile processes
– Equipment cleaning procedures
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.





Hi, what regulation/chapter cover periodic review of analytical methods please. Thank you