How to prepare for a GMP warehouse audit – tips and tricks
- Published on: Apr 29, 2023
What is a GMP warehouse audit and why is it important?
When we think of a pharmaceutical warehouse, we immediately visualise people working with vests and safety shoes, picking goods with forklifts from warehouse storage racks, lifting boxes from pallet to trolley, and receiving and despatching goods on the truck for distribution.
While all these activities fit common warehouse responsibilities, many more things are happening inside the GMP warehouse. With all the productive work being accomplished, mistakes and errors are common occurrences in the warehouse.
As we are talking about pharmaceutical and medical-grade products, there must not be any room for error, which can lead to significant patient safety.
Hence, frequent warehouse audits are critical to Good Manufacturing Practice (GMP).
Let’s look into a few commonly reported errors occurring in the GMP warehouse.
i. Inventory inaccuracies:
Inaccurate inventories are the biggest headaches for any warehouse.
43% of small businesses either don’t track inventory or use a manual method, contributing to inaccuracies. (Source: Wasp Barcode).
Inventory error can happen when the warehouse’s quantity or type of goods does not match the records or system data. This can lead to overstocking or stockouts, which can be costly for the business.
ii. Picking errors:
Picking errors occur when incorrect items are selected for an order.
The average picking error rates in the warehouse range between 1 and 3%. Studies show that a single picking error can reduce the profitability of an order by as much as 13%. (Source: 6 River Systems)
Which is a significant problem for businesses looking to increase productivity and profitability.
iii. Shipping errors:
These errors occur when incorrect items or quantities are shipped to the customer. This can result in returns and re-shipments, which can be costly for the business.
34% of businesses ship late because products are sold that are not actually in stock. (Source: The 2017 e-Commerce Fulfilment Report)
This can lead to incorrect or incomplete shipments, resulting in customer dissatisfaction and loss of revenue.
iv. Receiving errors:
Receiving errors occur when the goods received do not match the purchase order or packing list. This can lead to inventory inaccuracies and delays in processing orders.
Regarding warehouse inventory errors, human error is the top issue in 46% of warehouses (Source: Flexis).
Maintaining Good Warehouse Practice (GWP), employee training, warehouse record keeping, and frequent GMP warehouse audits can profoundly reduce most of these warehouse errors.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Understand the regulatory requirements for GMP warehouse audit
The first step in preparing for a warehouse audit is understanding the GMP requirements.
GMP regulations vary by country and the types of goods you process in the warehouse. So, it’s important to research the specific regulations for your facility and location.
Some standard GMP requirements include proper documentation and record-keeping, quality assurance, employee training and qualifications, facility cleanliness and maintenance, and good distribution practice.
Please make sure you have a thorough understanding of these requirements and how they apply to your warehouse operations.
As an example, a mix-up of sodium chloride and potassium chloride in the warehouse may be undetected by the laboratory but could have fatal consequences for patients injected with the wrong salt.
GMP rules require that there is extensive record keeping in the warehouse on receiving, sampling, testing, releasing, storing, and distributing of finished products for this very reason.
Without accurate and detailed records in the warehouse, it may be impossible to investigate a problem quickly or thoroughly when required. Any delay may cause additional problems for patients.
According to US FDA CFR211, Sec. 211.188 Batch production and control records.
“Batch production and control records shall be prepared for each batch of drug product produced and shall include complete information relating to the production and control of each batch”.
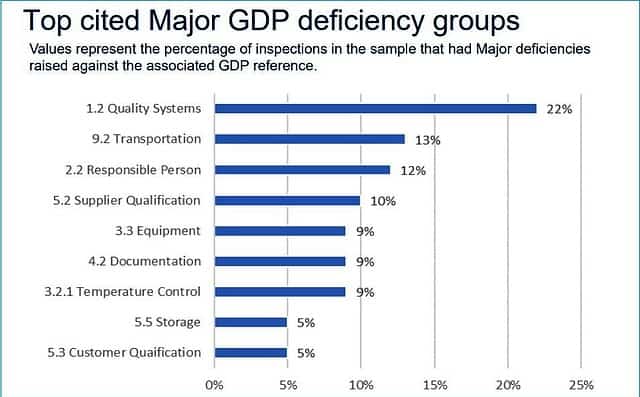
To raise your awareness, I have a list for you below of the top 10 warehouse deficiencies reported by the MHRA – UK in 2016, which indicates the industry at large.
How do you prepare for a GMP warehouse audit?
In the pharmaceutical industry, the warehouse is the facility responsible for procuring, holding, and supplying medicinal products to distribution facilities.
During the warehouse audit, you should focus on the overall health check of your warehouse activities against the Good Warehouse Practice (GWP) and Good Distribution Practice (GDP) guidance.
Typically, you should prepare for a GMP audit by following some or all of the areas listed below:
a. Understand the scope and objectives of the audit
Before facing a GMP warehouse audit, it’s essential to understand the scope and objectives of the audit. This will help you focus your efforts and ensure that you address the most critical areas of your warehouse operations.
In general, the following areas of your warehouse would be in the audit scope:
– Goods receipt,
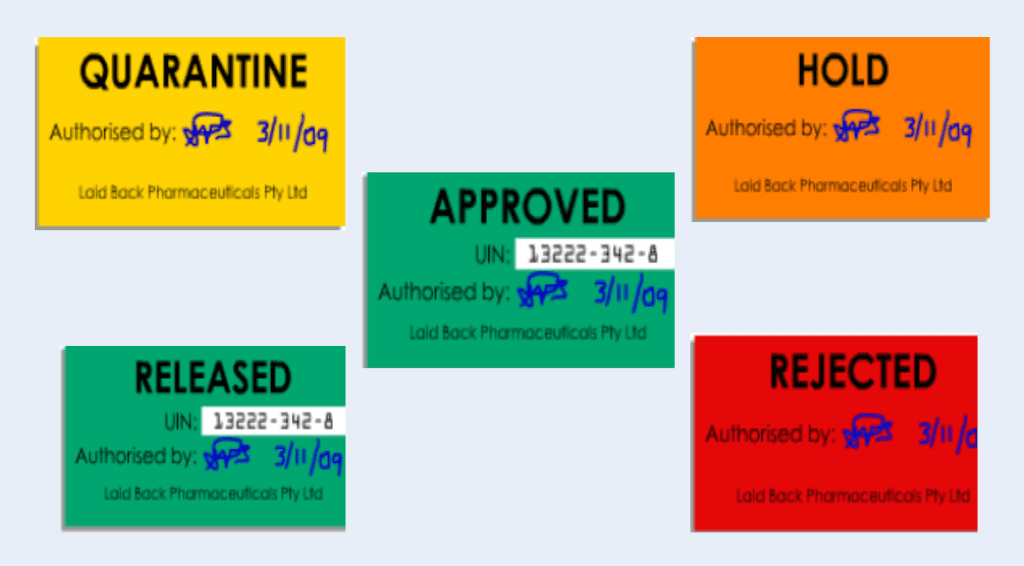
– Quarantine process,
– Sampling and release to production
– Inventory management
– Management of issues, returns, rejects, and reworks,
– Material and goods status control
– Temperature and humidity control
– Electronic systems in the warehouse e.g. ERP system
– Order fulfilment, and
– Storage and shipping process of cold chain products
– Pest control
– Documentation requirements of the warehouse and record system, etc.
Also, try to understand the objectives of the audit to help you prepare better:
– Compliance with GMP requirements
– Warehouse general housekeeping
– Identifying inefficiencies and non-conformances
– Possibilities of mix-ups and cross-contamination
– Improving customer satisfaction
With a proper understanding of the scope and objectives of the audit, you can ensure a high level of compliance in the audit.
b. Conduct regular self-audit
Before the actual GMP audit, it’s important to conduct a self-audit to identify any potential issues or areas of non-compliance.
This will give you time to take care of any issues before the actual audit and ensure that your facility complies with regulations.
Please review your documentation and record-keeping practices, employee training and qualifications, facility cleanliness and maintenance, sampling, storage, and material status control.
Could you make a checklist of any areas that need improvement and create an action plan to address them?
By conducting a self-audit, you’ll be better prepared for the actual GMP audit and increase your chances of passing with flying colours.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
c. Develop a comprehensive list of standard operating procedures (SOPs)
One of the most important steps in preparing for a GMP warehouse audit is to develop a comprehensive Standard Operating Procedure (SOP) for all aspects of warehouse operations.
This includes everything from receiving and storing materials to packaging and shipping finished products.
Your procedures should detail how to perform each activity, including who is responsible for each task, what equipment and materials are needed, and what documentation (e.g. forms, protocols, etc.) is required.
Please ensure your SOPs are up-to-date and reflect any recent changes in regulations or industry best practices.
A well-written and comprehensive SOP will demonstrate to auditors that you take compliance seriously and have a clear plan to ensure it.
Your list of SOPs should cover the following warehouse functions:
– Cleaning and maintenance of the premises,
– Pest control
– Receipt and checking of the deliveries
– Storage, including monitoring and, where necessary, control of the storage conditions
– Transportation conditions
– Management and control of road/air and sea transportation
– Stock security on site and in transit
– Cross-contamination control
– Withdrawal of stock from saleable distribution
– Records, including records of orders & distribution
– Handling returned and recalled products.
– Destruction of material
– Complaint handling
– Handling of suspected counterfeit products
– Management of personnel (including training)
– Self-inspections
d. Train your staff
Another critical aspect of preparing for a warehouse audit is ensuring all staff members are adequately trained and knowledgeable about GMP regulations and procedures.
This includes warehouse staff and anyone who may interact with products or materials in the facility, such as quality control personnel or maintenance workers.
Please make sure all staff members receive regular training on GMP requirements and understand the importance of compliance.
This will help you pass the audit and ensure your facility operates safely and efficiently.
e. Maintain accurate records
Maintaining accurate records is one of the most important aspects of preparing for a GMP warehouse audit.
This includes records of all incoming and outgoing products, as well as any quality control tests or inspections that are performed.
Make sure that all records are up-to-date and easily accessible and that they are kept in a secure location.
This will help you pass the audit and ensure that you can quickly and easily trace any issues or concerns that may arise in the future.
f. Review inventory management processes and accuracy
One of the key areas to focus on during a warehouse audit is inventory management.
This includes reviewing processes for receiving, storing, and tracking inventory and ensuring accuracy in inventory counts.
You can start by looking over your current inventory management system and finding any areas you’d like to improve.
This may include implementing barcode scanning technology, improving labelling and organisation, or updating your inventory tracking software.
Also, conduct a physical inventory count to ensure your records match the inventory.
By improving your inventory management processes and accuracy, you can reduce costs and improve customer satisfaction by ensuring that products are always in stock and ready to ship.
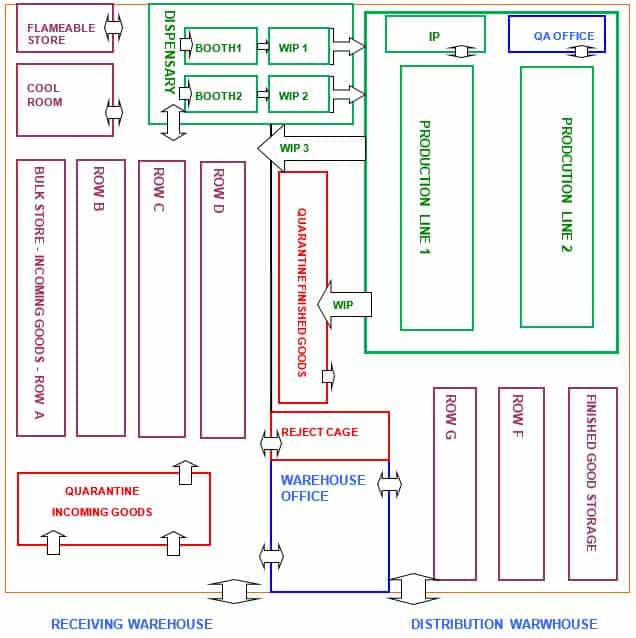
g. Assess warehouse layout and organisation
Another important aspect of preparing for a GMP warehouse audit is to understand the layout and organisation of your warehouse.
You should be aware that the material and personnel flow does not breach the requirements outlined in the layout.
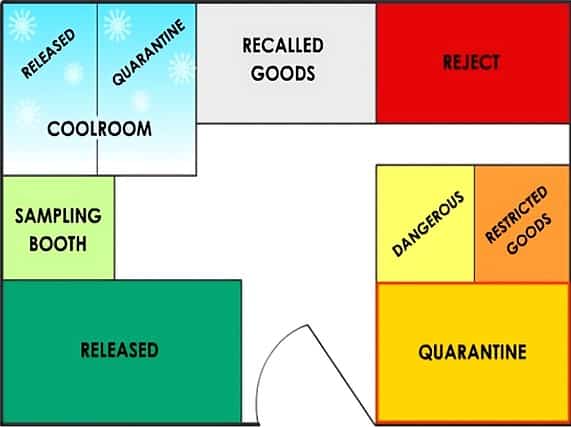
Before the audit, could you review the schematic diagram of the warehouse and production areas? From the diagram, clearly Identify the following areas are functionally organised:
– In-coming goods storage unit types and storage bin types,
– Quarantine areas
– Reject Cage
– Cool room
– Flammable storage
– Dispensary booths
– Production area
– Finished goods quarantine area and
– Finished goods storage areas before shipping
Review the flow of materials and personnel through the warehouse. Identify any bottlenecks or areas of concern e.g. potential for material mix-up.
Please ensure the warehouse has temperature mapping and can hold temperature and humidity-sensitive products.
Try our FREE online GMP Skill Booster tests. It’s challanging, it’s refreshing and it’s FREE. Try now!
h. Evaluate equipment and technology usage
In addition to assessing the layout and organisation of your warehouse, it’s important to evaluate the equipment and technology you are using.
This includes reviewing the condition and functionality of your forklifts, pallet jacks, conveyor belts, and other machinery.
Ensure all equipment is adequately maintained and serviced regularly to prevent breakdowns and delays.
Review the efficacy of any automated storage and retrieval systems or barcode scanning implemented in the warehouse.
i. Analyze safety and security protocols
Safety and security are critical components of any warehouse operation.
During the audit, your warehouse will be evaluated for safety and security compliance to ensure they are up-to-date and effective.
This includes reviewing emergency response plans, fire safety procedures, and security measures such as access control and surveillance systems.
You should also take a look at the condition of your equipment and machinery to make sure that they are safe to operate and do not have a risk to your employees.
Conduct regular housekeeping audits in the warehouse: A checklist approach
Together with other warehouse and QA staff, prepare a comprehensive set of questionnaires covering all areas of warehouse activities.
Prepare a schedule for self-audit and conduct the audits as scheduled. Keep an eye on non-conformances and implement effective corrective actions.
Please be sure to look for any adverse trend that is forming and address those concerns before the problem grows too big to control.
A typical warehouse audit checklist should include the following areas of concern:
a. Warehouse office areas
– Is documentation appropriately stored?
– Contact lists near phones are clearly displayed, current, and easily accessible.
– All personal items are stored in lockers.
b. Management of standard operating procedure (SOP)
– Are SOP files accessible and filed alphanumerically?
– Have the superseded versions been returned to QA?
c. Environmental, health and safety
Would you happen to know if all fire/emergency exits and stairs are clear?
– Safety signs are clearly visible
– Access paths are unimpeded
– Forklifts parked away from traffic flow with tines lowered and keys removed when unused.
– Fire extinguishers/hoses are visible and accessible.
– All fire extinguishers are within the calibration range.
– Floors are clear of spills, chemical residue, and excess materials.
– Emergency stop buttons are visible and accessible.
– No bin sheets on items
– The heaviest items are stored on lower shelves.
– All cardboard was removed, except where necessary for goods storage.
– Cardboard should be kept at a minimum level.
– Clear access to shelving
– Safety equipment worn and used for appropriate tasks (ear protection/safety glasses/gloves)
d. Staging and shrouded areas
– Area waste bins are not overflowing.
– Waste bins are used for appropriate materials only.
– Empty pallets are stored in designated areas and lying flat.
– The floor is free of equipment not in use.
– Floors are cleaned free of excess dust.
– No expired IPA bottles.
– All Roller doors are closed when not in use.
– Materials to be used for Shrouding are stored in an orderly manner.
– Pallets of Goods to be Shrouded correctly stored and labelled.
e. Flammable goods store
– The flammable store is locked when not in use. Keys are not left in the door.
– The area is used for storage of flammable goods only.
– No storage of goods on the floor.
– No waste in the area.
– IPA bottles are stored safely with correct labelling and within the expiry date.
f. Bulk store
– All components/products/packing materials are appropriately labelled and stored in allocated areas.
– All racking and storage areas are labelled clearly.
– All rejected stock is placed into the correct storage location.
– All batch numbers are separated and clearly labelled.
– All Roller doors are closed when not in use.
g. Waste area
– The ground area is free of waste.
– All waste is in the correct waste disposal area.
– Equipment is stored appropriately and does not block access ways.
– Waste from the laboratory is adequately labelled and segregated.
We have tried to provide some examples of warehouse locations and housekeeping audit checklists.
You should prepare your own questionnaire involving your team members who would know the warehouse better than anybody.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
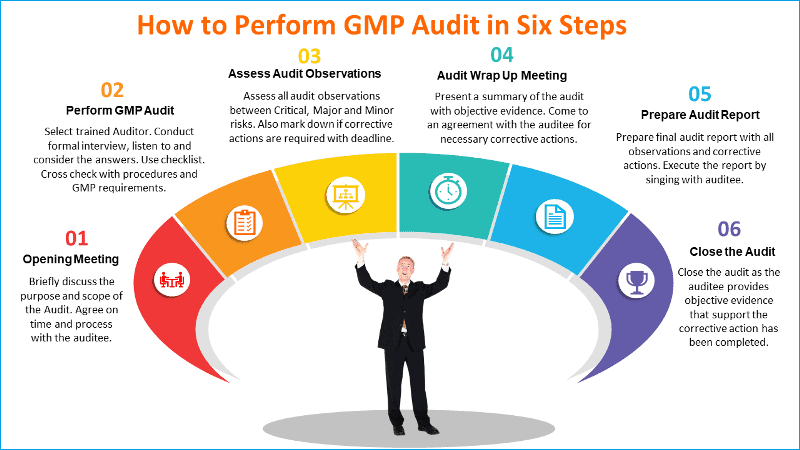
The role of the GMP auditor during warehouse audit?
An experienced GMP auditor is typically responsible for conducting official GMP warehouse audits.
The auditor should have a tertiary degree qualification and or certification from a recognised organisation.
Auditors should have strong knowledge and expertise in good warehouse practice, distribution practice, quality assurance, quality control, or regulatory affairs, especially concerning warehouse activities.
The GMP auditor should make adequate preparations before engaging in the warehouse audit.
Whether you are an auditor or an auditee responsible for warehouse management, the following list of audit preparatory activities can help make the warehouse audit an effective tool to eliminate errors.
a. Things GMP auditor should review before warehouse audit
1. Review all warehouse documentation and quality systems
– Find out what prescription drug products/medicinal products are at the site.
– Determine if any products have special storage or handling requirements, including controlled drugs.
– If available, review observations and corrective actions from previous regulatory inspections.
– Review observations and corrective actions from previous audits.
– Please look over the listed reference materials to make sure that you are familiar with the regulatory requirements.
– Obtain a list of reported product quality complaints.
b. Things GMP auditor should review during warehouse audit
1. Inspect the facility and equipment
– Tour the facility.
– Look for cracks and peeling paint in the ceiling, walls, and floor.
– Look for dust and dirt on racking and equipment.
– Review housekeeping logs for frequency of cleaning.
– Look for a secured quarantine area – administrative quarantine through a computerised system may be applied.
– Inspect the area used to store returned, outdated, and damaged drug products.
– Review pest control program, logs, and receipts.
– Review preventive maintenance program and logs.
– Ensure products are stored in accordance with their labelling.
– Ensure prescription drugs/medicinal products are stored apart from other products.
– Ensure controlled drugs, if any, are stored as required by legislation.
– Ensure Investigational Medicinal Products are stored separately.
– Review temperature & humidity charts and logs.
– Ensure probes are positioned to give representative values and as found during mapping.
– Review the calibration program.
– Review the security system for the facility; ensure the facility is secure from unauthorised entry.
– Review the alarm system and investigate if alarms are activated.
– Determine if the facility stores and distributes penicillin or other sensitizing agents and if adequate procedures are in place to contain spills and decontaminate the area.
– Determine if the facility stores in-process materials, raw materials, or excipients that are returned to the manufacturer for further processing.
– Determine if the firm has a control system for implementing changes to facilities and equipment, which includes reporting major modifications to the site.
2. Review the quality system
– Verify that there is an established and approved Quality System/Quality Policy that is endorsed by management.
– Determine if a representative has defined authority to implement and maintain the quality system.
– Verify that the quality system includes individual(s) with the authority to approve or reject procedures, specifications, and process changes that impact the quality of the finished products.
– If in the US, review all in-state and out-of-state authorised distributors/wholesale distributor licenses for the states where the wholesaler distributes.
– Verify that customers are authorised to receive prescription drug products/medicinal products before shipment.
– Review the disaster recovery plan and testing of the plan.
– Verify there is a written approved SOP for handling complaints.
– Review complaints.
– Review the wholesaler’s role in recalls.
– Determine if records are detailed enough to permit a recall.
– Determine if the wholesaler has been involved in recalls and that they have been carried out appropriately and in a timely manner.
– Verify that there is a system to handle deviations and that deviations are properly investigated.
– Verify there is a written approved SOP for change control.
– Review documentation of changes that have occurred.
– Could you verify that product quality complaints received with returned goods are forwarded to the site?
– Verify that an appropriate system for self-inspections has been implemented.
3. Ensure that warehouse staff have received adequate GMP training
– Verify that there is a documented training program.
– Review actual training documentation for specific employees.
– Verify that drug screens and criminal background checks were performed before hiring (US requirement).
– Verify that job-specific training is conducted and documented for employees.
– Could you verify that training is conducted with sufficient frequency to ensure that employees remain familiar with applicable regulations?
– Verify that there are clearly written job descriptions for employees.
– Verify that the training program incorporates requirements for temporary employees and -consultants.
– Review the list or organisation chart of Officers, Directors, Managers, and other persons in charge, including a description of their duties and a summary of their qualifications.
– Verify annual GMP/GDP training is performed.
– Verify that a record with an employee’s name, signature, and initials written by the employee exists.
4. Review the documentation system is adequate
– Verify that the firm has written approved SOPs (procedures) that include:
> Generation, change control, approval, and issuance of SOPs.
> Receipt of prescription drugs or medicinal products.
> Distribution of prescription drugs/medicinal products, including stop shipments, mis-shipments, and FIFO.
> Conducting an inventory of prescription medicinal products, including correcting all errors and inaccuracies.
> Storage of prescription drugs or Medicinal Products.
> Recall and/or withdraw prescription drugs or medicinal products from the marketplace.
> Identifying, recording, and reporting losses or thefts of prescription Drugs or medicinal products.
> Documenting deviations and notifying the site.
> Managing temperature-sensitive products.
> Managing penicillins or other sanitising agent spills.
– Review actual inventories performed.
– Review actual receiving records.
– Could you verify that there are procedures in place to ensure record retention for every receipt, distribution, or disposition of prescription drug product/medicinal product for the required retention time?
– Review loss/theft investigations and reports.
– Review the returned/damaged drug log and verify that the inventory received matches the inventory sent to a site-approved reverse distributor.
– Ensure that computer systems maintaining drug inventory and distribution records are validated and subject to change control.
– Ensure that computer systems are controlled to prevent diversion and theft of drug inventory.
– Review the management and control of road, air, and sea transportation
– Ensure that products are transported in a secure manner e.g. Box/hard-sided vehicles for road transportation.
– Ensure that if the authorised distributor/wholesale distributor distributes controlled substances, they are following the legal requirements
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
c. Things you should be aware of during the warehouse audit
– Senior management should be present at the opening and closing meetings of a warehouse audit.
– It is suggested that the senior management give a brief introductory presentation to the GMP auditor covering the department, function, or site being audited.
– Auditors should be accompanied at all times to meet the organisation’s WHS requirements and to facilitate the provision of documents, information, and movement through the facility.
– You must answer the auditor’s questions truthfully and honestly, in the most direct manner, to ensure prompt provision of information and adherence to the audit schedule timeliness.
– You should provide proper Induction to auditors for audited areas if they request entrance into restricted warehouse areas.
– You should request summary sessions (daily wrap-up meetings) from the auditors at the end of each day’s activities to clarify any issues that may have been unclear during the audit.
– Inform the auditor of any immediate corrective actions completed and take the opportunity to discuss the next day’s auditing program.
– You should communicate a short summary of significant issues or concerns raised each day of the audit to site management.
d. Audit wrap-up and reporting
– The auditor should provide a final wrap-up session by the end of the warehouse audit.
– During this session, you should agree on all written observations and verbal comments between the two of you.
– The auditor should provide clarification relating to observations or comments. Any misunderstanding during the audit should be resolved before the meeting ends.
– You should provide written responses with corrective actions to each observation and submit the response within an agreed timeframe.
– You and the GMP auditor should sign the audit closure report for future reference.
Conclusion
Preparing for a GMP warehouse audit can be a daunting task.
However, with the right approach, it can be proven to be an excellent opportunity to identify potential issues and improve processes.
The key to success is proactively identifying and addressing potential issues before they are discovered during an audit.
One way to do this is to conduct regular self-audits. An audit checklist approach can make the process consistent and less cumbersome.
Once issues and any adverse trends are identified, it is important to take corrective actions to address them.
When you prepare, you should also ensure that standard procedures and records are in place to comply with GMP regulations.
To prepare for a GMP warehouse audit, we recommend you take the following strategies:
– Organize all documentation and ensure that they are readily available
– Maintain cleanliness and orderliness in the warehouse
– Ensure that equipment is properly calibrated and maintained
– Train staff on GMP regulations and procedures to ensure that they are knowledgeable and prepared for an audit
– Prepare a checklist of items to be addressed during the audit
– Assign specific roles and responsibilities to staff members to ensure that all areas are covered
– Address any issues identified during the audit on time
– Continuously improve processes and procedures to maintain compliance with GMP regulations.
I think following this approach can better prepare you for a GMP warehouse audit. you can use the audit experience as an opportunity to improve your processes.
By being proactive and continuously improving processes and procedures, you can maintain your warehouse in compliance with GMP regulations and ensure the safety and efficacy of pharmaceutical products.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.
Related Posts
Six steps procedure for corrective and preventive action
What is acceptable quality limit and how to use AQL in sampling
Seven steps to complete a supplier selection process in GMP




I need a contact point for this site
I have been trying to reach out to you with no chance of success
I yolla, sorry for inconvenience. I have sent a private message.