Biological Test Methods Validation in Pharmaceuticals
- Published on: Oct 27, 2017
The following aticle will address the Validation practices of non-compendial or alternate Biological Test methods and the Verification of Compendial biological Test Methods to demonstrate that a test method is suitable for its intended purpose.
Biological test methods must be developed, documented and have sufficient detail so that a Qualified Laboratory Analyst can accurately perform the test in an environment suitable for the Test Methods and using the required Test Methods materials. All Test Methods shall be maintained under change control.
The scope of this article applies to all GMP operation responsible for the validation of biological test methods associated with products in or intended for the marketplace including testing
performed for release or stability evaluation of:
- Regulatory Starting Materials,
- Active Pharmaceutical Ingredients (API),
- Intermediates,
- In-Process Materials,
- Drug Products,
- Biologics,
- Medical Devices,and
- Raw Materials (RM).
Biological Test Methods shall be validated unless the method employed is included in the current edition of an official pharmacopoeia (e.g., EP, JP, or USP) or other recognized standard references. Verification of a compendial biological test method must demonstrate that the test method is suitable for its intended purpose.
This guidance applies to biological test methods. The following are examples of biological test methods:
- Microbiological assay of antibiotics;
- Potency Bioassays (e.g., vitamins);
- Immunochemical assays [e.g. Enzyme Linked Immunosorbent Assay (ELISA)];
- Virus titration; and
- In vivo and in vitro (cell-based) biological assays.
Validation of a Bacterial Endotoxin test (BET) is not addressed in this article.
Specificity must be demonstrated for verification of all compendial (e.g., EP, JP, USP) biological test methods under actual use conditions in the laboratory, including any minor
variations in the Test Methods.
Non-Compendial or Alternate Biological Test Methods shall be validated and must be shown to be equivalent to the compendial or regulatory filing method. Validation shall include consideration of the following validation characteristics applicable to the method:
- Accuracy,
- Precision,
- Repeatability,
- Intermediate Precision (intra-laboratory), and
- Reproducibility (inter-laboratory),
- Specificity, and
- Quantitation Limit (QL).
Robustness of the biological test method shall be considered during method validation if not addressed during method development.
Validated alternate biological test methods shall be submitted to the Regulatory Authority, when required, prior to implementation.
Biological Test Method Validation or Verification Studies shall be performed and documented according to an Approved Protocol that defines the parameters being evaluated, the acceptance criteria for each parameter and the Test Methods. The protocol must be approved by the Laboratory Manager prior to execution of the validation studies.
A Method Validation or Verification Report shall be prepared that documents the results of the validation or verification study, including the evaluation of each parameter and comparison against acceptance criteria. Any Deviations from the protocol must be documented and the impact of the deviations discussed in the report.
Qualified Personnel shall be identified with the following roles and responsibilities in regards to biological test method validation or verification:
The Laboratory Manager at the Site conducting the validation or verification is responsible for assuring that the biological methods are validated
or verified and for reviewing and approving the Final Report; and
The Quality Authority, independent of the Laboratory Manager, at the Site conducting the validation or verification study is responsible for
reviewing and approving the final report for compliance with applicable Site policies and procedures.
Automated Systems used as a part of a biological test method and Computerized Laboratory Systems shall include Validation of the computerized system. Qualified Personnel shall perform:
- Validation studies, and
- Verification of compendial methods.
Written and Approved Procedures shall describe the preparation, Laboratory labelling, storage, and use of laboratory Reagents, solutions, buffers and Reference Standards.
Test Equipment used in the execution of the biological test method protocol must be qualified and have a current Calibration status.
Biological Test Method Validation and Verification Studies shall be documented and retained in accordance with site record retention requirements.
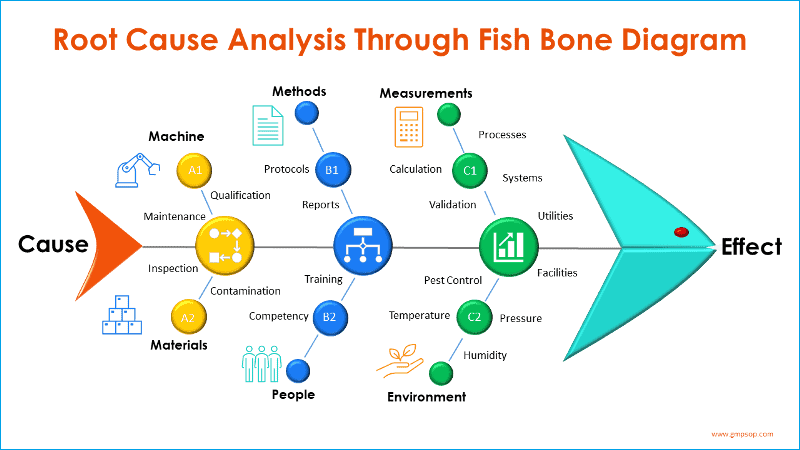
The Need for and Degree of Revalidation shall be determined by the Laboratory Manager. Changes to be considered in determining the need for and extent of revalidation include, and are not limited to:
-
- Changes to the biological test method (e.g., new or modified sample preparation procedure, change to detection conditions, change to
instrument settings and/or operating conditions); - Changes to the sample being tested (e.g., process change for API or change in
- Changes to the biological test method (e.g., new or modified sample preparation procedure, change to detection conditions, change to
composition of the product); or
- Changes in compendial or regulatory requirements (e.g., ICH, EMEA).
Legacy Methods – the need to validate a legacy biological method that has not been previously validated or is not a compendial method shall be based on an impact assessment by the responsible Site Quality Team. Legacy methods shall be assessed against this guidance to ensure available method documentation is current and reflects the method. A Retrospective Validation approach may be used to support the biological method validation.