How to perform operational qualification – step by step
- Published on: Jul 31, 2023
Importance of operational qualification in regulated industries?
Operational qualification is essential for guaranteeing the safety, performance, and reliability of critical equipment and systems in regulated industries like biotechnology, pharmaceuticals, and medical devices.
It is a methodical process that confirms and records that every important part of equipment operates as intended and meets user expectations.
Your company can reduce risks, avoid expensive mistakes, and preserve the integrity of its processes by undertaking operational qualifications.
Equipment or system with inconsistent performance can have major repercussions on pharmaceutical products, including faulty products, market recalls, and other regulatory non-compliance.
When you will conduct an operational qualification successfully you can be benefitted by:
i. Ensuring that the equipment is operating within the range, and functions as intended, minimizing the risk of equipment-related issues that could have impacted product quality.
ii. Helping your company meet the regulatory requirements set by organizations like the FDA, EMA, and other relevant authorities.
iii. Identifying and mitigating potential risks associated with equipment operation, preventing costly failures and downtime.
iv. Providing a comprehensive and detailed record of the operational parameters, and facilitating future audits and inspections.
v. Improving productivity since the equipment that is operating correctly is more likely to operate efficiently, and reduced operational costs.
Successful OQ provides confidence in the reliability and accuracy of the equipment, contributing to the overall quality and safety of pharmaceutical products.
What are the FDA requirements for operational qualification?
The FDA sets stringent requirements for operational qualification for critical equipment and systems to ensure the safety and efficacy of pharmaceutical products.
These requirements mandate that pharmaceutical companies thoroughly document OQ protocols, testing methods, acceptance criteria, and results.
The FDA emphasizes risk-based approaches and expects your company to address critical equipment and potential failure modes during OQ.
You must conduct operational qualifications in compliance with the rules and regulations developed by regulatory agencies like the Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Regulatory agencies have provided guidelines, qualification outlines together with the minimum standards to maintain. You must follow these guidelines and reporting requirements during operational qualification at the minimum.
Regulatory requirements require you to create and adhere to a validation master plan that describes the strategy, obligations, and processes for operational qualification.
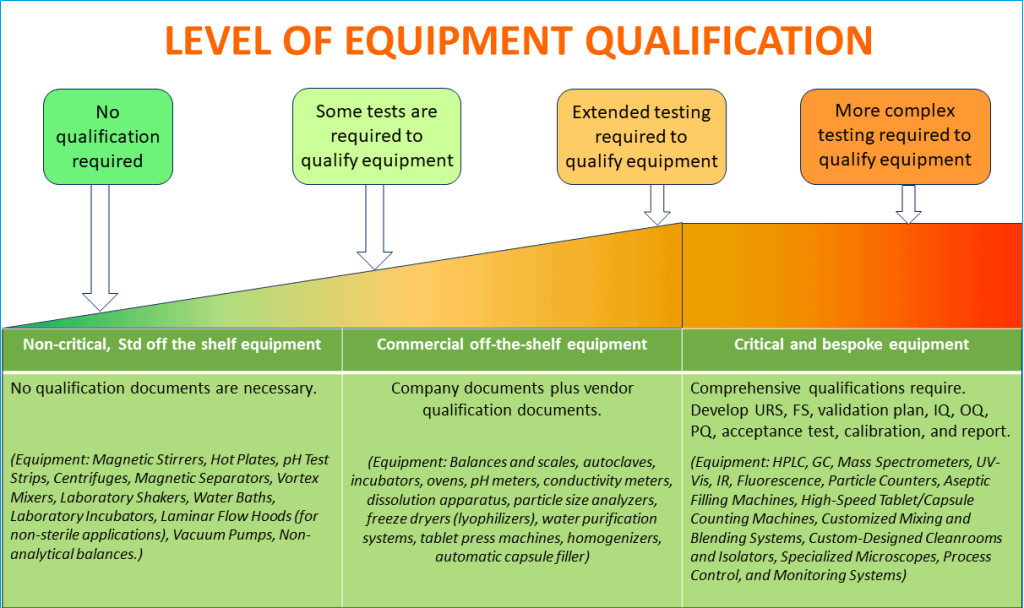
When is equipment qualification necessary?
Pharmaceutical manufacturing operations typically maintain equipment varying from simple balances and scales to complex automated process control and monitoring systems.
Therefore, it is not practical for you to apply a single set of principles to justify the same level of qualification program, including operational qualification with such dissimilar equipment. Also, it would be scientifically inappropriate.
You should conduct a risk-based categorization of all equipment and systems and list them between,
– Non-Critical
– Commercial off the shelf (COTS)
– Critical, and
– Bespoke
When you are deciding which equipment needs qualification testing, follow the GAMP (Good Automated Manufacturing Practice) categorization guidelines developed by the International Society for Pharmaceutical Engineering (ISPE).
GAMP provides a framework for the validation of automated systems. GAMP categorizes software and hardware based on their criticality and complexity, which helps to determine the level of qualification effort required.
GAMP divides equipment and systems into five categories:
Category 1: Infrastructure Software
These are foundational computerized systems, such as operating systems, database management systems, and network infrastructure.
These components typically have a lower impact on product quality and patient safety. Therefore, these are considered to be non-critical.
Category 2: Non-configurable Software
Non-configurable software includes commercial off-the-shelf (COTS) applications that are used without any customization or modification.
For example, standard office software like word processors and spreadsheet applications.
While GAMP 2 systems are not directly involved in pharmaceutical processes, they may handle data that needs to be validated.
Category 3: Configurable Software
Configurable software is customizable to some extent but does not require full software development.
Laboratory information management systems (LIMS) and data acquisition systems are the best examples.
Category 4: Bespoke Software
Bespoke software refers to custom-developed software specifically designed to meet unique requirements and processes within a pharmaceutical operation.
These systems are often critical to product quality and safety and require more extensive validation efforts.
Category 5: Process Control Systems
Process control systems are complex systems responsible for controlling and automating manufacturing processes in pharmaceutical facilities.
These systems have a direct impact on product quality and safety and are considered the most critical and complex to validate.
Follow the diagram below to make a decision on the extent of equipment qualification activities that are necessary based on the product risk and criticality of equipment operation on products.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
A good idea is to review the GAMP categorization and assess criticality of equipment on product quality.
Then classify all of your equipment and systems as below. This will facilitate making an informed decision on how many qualification activities will be necessary.
i. Non-Critical (GAMP Category 1):
– Laboratory Glassware (Beakers, Flasks, Pipettes)
– Magnetic Stirrers
– Hot Plates
– pH Test Strips
– Safety Equipment (Laboratory Coats, Safety Glasses)
– General Purpose Centrifuges
– Magnetic Separators
– Vortex Mixers
– Laboratory Shakers
– Water Baths
– Laboratory Incubators
– Laboratory Freezers (Non-critical storage)
– General Purpose Ovens
– General Utility Carts
– Laminar Flow Hoods (for non-sterile applications)
– Vacuum Pumps (for non-critical applications)
– Non-Sterile Sample Containers
– Standard Laboratory Balances (Non-analytical balances)
ii. Commercial off the shelf – COTS (GAMP Category 2 & 3):
– Balances and Scales
– Autoclaves
– Incubators
– Ovens
– pH Meters
– Conductivity Meters
– HPLC Systems
– UV-Visible Spectrophotometers
– Dissolution Testing Apparatus
– Particle Size Analyzers
– Freeze Dryers (Lyophilizers)
– Water Purification Systems
– Tablet Press Machines
– Homogenizers
– Refrigerators
– Freezers
– Environmental Monitoring Systems
– Automatic Capsule Filling Machines
iii. Critical & Bespoke (GAMP Category 4 & 5):
– High-Performance Liquid Chromatography (HPLC) Systems (Critical for analytical testing and product quality assessment).
– Gas Chromatography (GC) Systems (Critical for analyzing volatile compounds).
– Mass Spectrometers (Critical for molecular identification and structural analysis).
– Spectrophotometers (UV-Vis, IR, Fluorescence) (Critical for quantitative analysis and drug release testing).
– Particle Counters (Critical for monitoring and controlling cleanroom environments).
– Aseptic Filling Machines (Critical for sterile product filling to maintain product integrity and patient safety).
– Lyophilizers (Critical for freeze-drying temperature-sensitive pharmaceutical products).
– Sterilization Autoclaves (Critical for ensuring aseptic conditions and product safety).
– Disintegration Testers (Critical for assessing the disintegration properties of solid dosage forms).
– High-Speed Tablet/Capsule Counting Machines (Critical for accurate counting and packaging of tablets and capsules).
iv. Bespoke systems:
– Customized Mixing and Blending Systems (Designed for specific formulations or challenging mixing requirements).
– Custom-built Reactors and Fermenters (For specialized pharmaceutical synthesis and bioprocessing).
– Custom Analytical Instruments (Developed for unique analytical methods or specific sample types).
– Custom-Designed Cleanrooms and Isolators (For specialized aseptic manufacturing and containment).
– Automated Robotic Systems for Sample Handling (Designed to automate complex sample preparation and handling).
– Specialized Microscopes (For unique inspection and analysis tasks in pharmaceutical research).
– Custom Temperature and Humidity Chambers (For stability testing under specific conditions).
– Process Analytical Technology (PAT) Systems (Customized for real-time monitoring and control of manufacturing processes).
– Customized Dissolution Testing Apparatus (Tailored for specific dosage forms or release rate requirements).
– Process Control and Monitoring Systems (Designed for specific manufacturing processes and data collection needs).
What isn't covered in operational qualification?
Operational qualification simply focuses on the functionality and performance of equipment. It does not address other validation steps.
For example, OQ does not address installation qualification (IQ), which verifies that the equipment is installed correctly and in compliance with design specifications.
Similarly, OQ is distinct from performance qualification (PQ), which evaluates equipment under actual operating conditions using real products or processes.
Installation qualification vs. operational qualification
Installation qualification (IQ) and operational qualification (OQ) are two distinct qualification testing phases with different objectives.
IQ focuses on verifying that the equipment is properly installed, calibrated, and connected as per design specifications.
Installation qualification verifies and documents that all critical aspects of equipment installation adhere to predetermined specifications and manufacturer’s recommendations.
On the other hand, operational qualification evaluates the equipment’s functional performance within its intended operating range.
In terms of the testing sequence, you have to perform IQ prior to OQ verification.
Nevertheless, when you will devise an installation qualification protocol it is a common practice to include performance qualification tests with it.
One of the primary reasons for conducting both IQ and OQ side by side is that they are interrelated. They can be performed using the same test protocol. But their objectives are different.
That will provide you with an upper hand in merging the tests, troubleshooting, and final reporting of the verifications.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Difference between operational qualification (OQ) and performance qualification (PQ)
Operational qualification (OQ) ensures that the equipment operates according to its design parameters, while PQ evaluates the equipment’s performance using real products or processes.
In terms of the testing sequence OQ precedes PQ, and both are essential for demonstrating equipment suitability and reliability on manufacturing operation.
Operational qualification examples in pharma
Let’s explore some practical examples of operational qualification tests required for critical equipment used in pharmaceuticals including their acceptance criteria.
Operational qualification of an autoclave
An autoclave is critical for the sterilization process in the pharmaceutical industry.
Operational qualification of an autoclave involves testing its temperature, pressure, and cycle times to ensure effective sterilization of equipment and materials.
1. Chamber temperature uniformity test:
Acceptance Criteria: The temperature difference between the hottest and coldest points within the chamber should not exceed a specified limit (e.g., ±2°C).
2. Heat distribution test:
Acceptance Criteria: The autoclave should uniformly distribute heat throughout the chamber, and temperature variations should not exceed a defined range (e.g., ±3°C).
3. Temperature accuracy test:
Acceptance Criteria: The temperature displayed by the autoclave’s controller should match the set temperature within a specified tolerance (e.g., ±1°C).
4. Overheat protection test:
Acceptance Criteria: The autoclave should activate the overheat protection system and shut down if the temperature exceeds a predefined limit (e.g., 2°C above the set temperature).
5. Vacuum leak test:
Acceptance Criteria: The autoclave should achieve and maintain the required vacuum level without significant pressure drop during the specified time (e.g., 27 inHg for a minimum of 5 minutes).
6. Bowie-Dick test (for Vacuum Autoclaves):
Acceptance Criteria: The Bowie-Dick test should show uniform steam penetration throughout the test pack, and no air pockets or wet spots should be present.
7. Biological indicator test:
Acceptance Criteria: The biological indicators should demonstrate a specified reduction in spore survival, indicating the effectiveness of sterilization (e.g., no growth or a certain percentage reduction).
8. Load configuration test:
Acceptance Criteria: The autoclave should be able to accommodate the intended load size and configuration while maintaining proper sterilization conditions.
9. Cycle time test:
Acceptance Criteria: The sterilization cycle should complete within the specified time limits without exceeding the defined duration.
10. Pressure and vacuum rate test:
Acceptance Criteria: The autoclave should achieve and maintain the required pressure and vacuum levels within specified timeframes during different phases of the sterilization cycle.
11. Alarm and safety test:
Acceptance Criteria: The autoclave’s alarms should activate and safety interlocks should function correctly when triggered by predefined events or conditions.
Operational qualification of a tablet compression machine
You can use some or all the tests during the operational qualification of tablet compression machine depending on the manufacturer’s specifications.
1. Weight Variation Test:
Acceptance Criteria: The tablet weight variation should be within a specified percentage range (e.g., ±5% of the average weight).
2. Hardness Test:
Acceptance Criteria: The tablet hardness should be within the defined range (e.g., X Newtons to Y Newtons) to ensure proper mechanical strength.
3. Thickness Test:
Acceptance Criteria: The tablet thickness should fall within the specified tolerance (e.g., ±0.1 mm) to ensure consistent tablet dimensions.
4. Friability Test:
Acceptance Criteria: The friability of tablets should not exceed a certain percentage (e.g., ≤1%) to ensure tablet durability during handling.
5. Disintegration Test:
Acceptance Criteria: Tablets should disintegrate within a defined time period (e.g., ≤15 minutes) under specified conditions.
6. Content Uniformity Test:
Acceptance Criteria: The active ingredient’s content within the tablets should be within a specified percentage range (e.g., 90% to 110% of the label claim).
7. Tablet Identification Test:
Acceptance Criteria: Tablets should have clear and accurate identification markings, such as logo, product code, and strength.
8. Compression Force Variation Test:
Acceptance Criteria: The compression force applied during tablet compression should remain consistent within a specified range (e.g., ±5% of the average force).
9. Output Rate Test:
Acceptance Criteria: The tablet compression machine should achieve the required output rate (tablets per minute) as per production requirements.
10. Lubrication Effectiveness Test:
Acceptance Criteria: The lubrication system should effectively reduce friction during compression, ensuring smooth tablet ejection.
11. Machine Calibration Verification:
Acceptance Criteria: Calibration verification should confirm that the tablet compression machine’s calibration is accurate and up-to-date.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Operational qualification of a purified water system
A purified water system is critical for producing high-quality water for pharmaceutical processes.
1. Conductivity Test:
Acceptance Criteria: The conductivity of the purified water should be within the specified range (e.g., X μS/cm to Y μS/cm) to indicate the level of impurities.
2. Total Organic Carbon (TOC) Test:
Acceptance Criteria: The TOC levels in the purified water should be within the defined limit (e.g., ≤ 500 ppb) to ensure the absence of organic contaminants.
3. Microbial Enumeration Test:
Acceptance Criteria: The microbial count in the purified water should be below a specified threshold (e.g., ≤ 10 CFU/100 mL) to demonstrate microbial control.
4. Endotoxin (LAL) Test:
Acceptance Criteria: The endotoxin levels in the purified water should be below the acceptable limit (e.g., ≤ 0.25 EU/mL) to ensure the absence of pyrogens.
5. pH Measurement Test:
Acceptance Criteria: The pH of the purified water should be within the predefined range (e.g., 6.0 to 7.5) for compatibility with the intended applications.
6. Temperature Control Test:
Acceptance Criteria: The temperature of the purified water should be maintained within the specified range (e.g., ±2°C) to ensure consistent water quality.
7. Water Flow Rate Test:
Acceptance Criteria: The water flow rate from the purified water system should meet the required specifications (e.g., X liters per minute).
8. Recovery Test:
Acceptance Criteria: The purified water system’s recovery test should demonstrate that the system can recover promptly after temporary shutdowns or disturbances.
9. Leak Test:
Acceptance Criteria: The purified water system should pass a leak test to ensure there are no leaks in the system, preventing contamination and water wastage.
10. Power Failure Test:
Acceptance Criteria: The purified water system should be able to shut down and restart appropriately during a simulated power failure without compromising water quality.
Operational qualification for HPLC instrument in laboratory
High-Performance Liquid Chromatography (HPLC) instruments are widely used for pharmaceutical product analysis including identification, assay, purity, and related substance.
Most often the HPLC system comes with configurable software which regulates the instrument’s performance. Sometimes, the interface is coupled with Laboratory Information Management System.
Therefore, the HPLC is a critical system and must be included for operational qualification.
1. System Suitability Test:
Acceptance Criteria: The system suitability parameters (e.g., resolution, tailing factor, and theoretical plates) should meet predefined acceptance limits to ensure the instrument’s performance is within specification.
2. Retention Time Precision Test:
Acceptance Criteria: The retention times of replicate injections of a standard solution should have a low relative standard deviation (e.g., ≤ 2%) to demonstrate precise retention time measurement.
3. Injection Volume Accuracy Test:
Acceptance Criteria: The measured injection volume should be within a specified percentage deviation (e.g., ±2%) from the target injection volume.
4. Flow Rate Accuracy Test:
Acceptance Criteria: The measured flow rate should be within a defined tolerance (e.g., ±2%) of the set flow rate.
5. Wavelength Accuracy Test:
Acceptance Criteria: The measured wavelength of the detector should be within a specified deviation (e.g., ±1 nm) of the set wavelength.
6. Detector Linearity Test:
Acceptance Criteria: The detector response to a series of standard solutions of varying concentration should show a linear relationship with concentration (e.g., R² ≥ 0.995).
7. Limit of Detection (LOD) Test:
Acceptance Criteria: The HPLC instrument should be able to detect a known low concentration of analyte with a signal-to-noise ratio of at least 3:1.
8. Limit of Quantification (LOQ) Test:
Acceptance Criteria: The HPLC instrument should be able to quantify a known low concentration of analyte with a signal-to-noise ratio of at least 10:1.
9. System Precision Test:
Acceptance Criteria: The relative standard deviation (RSD) of replicate injections of a sample solution should be within acceptable limits (e.g., ≤ 2%) to demonstrate precision.
10. System Accuracy Test:
Acceptance Criteria: The measured concentration of a known standard solution should be within a specified percentage deviation (e.g., ±2%) from the expected value.
11. Gradient Performance Test:
Acceptance Criteria: The instrument should accurately and reproducibly deliver the programmed gradient profile during a gradient elution analysis.
12. Data System Verification:
Acceptance Criteria: The chromatography data system (CDS) should correctly record, process, and store analytical data from the HPLC instrument.
Operational qualification of HVAC system
Some qualification tests and acceptance criteria are listed below.
1. Temperature Mapping:
Acceptance Criteria: The temperature distribution within critical areas of the facility (e.g., manufacturing areas, storage areas) should be within the specified range (e.g., ±2°C) to ensure uniformity.
2. Relative Humidity Mapping:
Acceptance Criteria: The relative humidity levels within critical areas of the facility should be within the specified range (e.g., ±5%) to maintain appropriate environmental conditions.
3. Air Velocity Measurement:
Acceptance Criteria: Air velocity measurements should be within the defined limits (e.g., X to Y meters per second) to ensure proper air movement and ventilation.
4. Differential Pressure Testing:
Acceptance Criteria: Differential pressure measurements between critical areas (e.g., cleanrooms and adjacent spaces) should meet the defined pressure differentials to prevent cross-contamination.
5. Filter Integrity Testing:
Acceptance Criteria: HEPA filters should pass the integrity test, indicating that they are free from leaks and can effectively remove particulate contaminants.
6. Recovery Testing:
Acceptance Criteria: After a defined disruption (e.g., door opening), the HVAC system should be able to recover and restore the environmental conditions within an acceptable time frame.
7. Alarm and Safety Test:
Acceptance Criteria: The HVAC system’s alarms and safety features should activate as intended when specific parameters deviate from acceptable limits.
8. Smoke Visualization Test:
Acceptance Criteria: In case of a smoke visualization test, the HVAC system should maintain the intended air movement and avoid smoke infiltration into critical areas.
9. Air Exchange Rate Verification:
Acceptance Criteria: The air exchange rate in critical areas should meet the specified frequency (e.g., X air changes per hour) to ensure adequate ventilation.
10. Recovery Time Verification:
Acceptance Criteria: The HVAC system should be able to recover from power failures or temporary shutdowns and restore the required environmental conditions within an acceptable time frame.
11. Airflow Direction Verification:
Acceptance Criteria: The airflow direction in cleanrooms and controlled areas should follow the intended design, ensuring that contaminants are not recirculated or redistributed.
Operational qualification of software
Following are some examples of operational qualification tests and acceptance criteria for the validation of a pharmaceutical PLC (Programmable Logic Controller):
1. Software Requirements Verification:
Acceptance Criteria: The PLC software should meet all specified functional and technical requirements as documented in the software requirements specification (SRS) or user requirements specification (URS).
2. Code Review and Verification:
Acceptance Criteria: The PLC software code should be reviewed for accuracy, compliance with coding standards, and adherence to best practices.
3. Installation:
Acceptance Criteria: The PLC software should be installed correctly, and all required components should be present and properly configured.
4. Backup and Restore:
Acceptance Criteria: Backup and restore procedures for the PLC software should be tested to ensure data recovery in case of system failures or data loss.
5. Security:
Acceptance Criteria: Access controls and security measures should be implemented to prevent unauthorized access and modifications to the PLC software.
6. Change Management:
Acceptance Criteria: Change management procedures for the PLC software should be in place to control and document software changes.
7. Alarm and Event Handling:
Acceptance Criteria: Alarms and events generated by the PLC software should be tested to ensure they are appropriately activated and trigger the desired actions.
8. Failure Modes Testing:
Acceptance Criteria: Failure modes of the PLC software should be tested to assess how the system responds to abnormal conditions or errors.
9. Performance Testing:
Acceptance Criteria: The PLC software’s performance should be validated under different operating conditions to ensure it can handle the required workload efficiently.
10. Integration Testing:
Acceptance Criteria: The PLC software should be integrated and tested with other control systems and equipment to verify proper communication and coordination.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Operational qualification for pipework
Following are some examples of operational qualification tests and acceptance criteria for the validation of pipework used in pharmaceuticals:
1. Material Verification:
Acceptance Criteria: The materials used for pharmaceutical pipework should be verified to be compatible with the intended process fluids and should meet regulatory requirements.
2. Dimensional Check:
Acceptance Criteria: The pipework dimensions should be measured and verified to ensure they comply with the specified design requirements.
3. Pressure Testing:
Acceptance Criteria: The pipework system should undergo pressure testing to verify its ability to withstand the specified operating pressure without leaks or failures.
4. Leak Testing:
Acceptance Criteria: The pipework system should be tested for leaks using appropriate methods, and the leak rate should be within acceptable limits.
5. Flushing and Cleaning:
Acceptance Criteria: Pipework should be thoroughly flushed and cleaned to remove any contaminants or debris before being put into service.
6. Sterilization Validation:
Acceptance Criteria: For critical pipework used in aseptic processes, sterilization validation should be performed to demonstrate the ability to achieve the required level of sterility.
7. Weld Integrity Testing (if applicable):
Acceptance Criteria: Welded joints in stainless steel pipework should be tested for integrity to ensure proper sealing and strength.
8. Cleaning-in-Place (CIP) Validation:
Acceptance Criteria: If CIP is used to clean the pipework, the effectiveness of the CIP process in removing residues and ensuring cleanliness should be validated.
9. Drain ability Verification:
Acceptance Criteria: The pipework should be designed and installed to allow proper draining to prevent liquid pooling and microbial growth.
10. Dead leg Assessment:
Acceptance Criteria: Dead legs (sections of pipework with no flow) should be minimized, and their impact on system hygiene and cleaning should be assessed.
11. Material Traceability:
Acceptance Criteria: The pipework should be labeled and documented with proper material traceability information, including material certificates and batch numbers.
12. System Integration:
Acceptance Criteria: The pipework system should be properly integrated with other equipment and systems, ensuring smooth flow and compatibility.
Operational qualification for utilities
Following are some examples of operational qualification tests and acceptance criteria for common utilities (other than HVAC and purified water system):
1. Clean Compressed Air System:
Acceptance Criteria: The compressed air system should meet the specified air quality standards, including the absence of oil, moisture, and particulate contaminants.
2. Steam System:
Acceptance Criteria: The steam system should provide steam of the appropriate quality, meeting standards for pressure, temperature, and purity.
3. Waste Management System:
Acceptance Criteria: The waste management system should ensure the safe disposal of pharmaceutical waste materials in compliance with environmental regulations.
4. Power Backup and Emergency Systems:
Acceptance Criteria: Power backup systems, such as generators and uninterruptible power supply (UPS), should function correctly during power outages to ensure continuous operations and data integrity.
5. Calibration of Instrumentation and Sensors:
Acceptance Criteria: Instruments and sensors used in utility systems should be calibrated and show accuracy within specified limits.
6. Alarm and Monitoring Systems:
Acceptance Criteria: Alarm and monitoring systems for utilities should be tested to ensure they detect deviations from defined parameters and activate appropriate alerts.
7. Gas Supply System (e.g., Oxygen, Carbon Dioxide):
Acceptance Criteria: The gas supply system should deliver gases of the specified purity and at the required flow rates without contamination or fluctuations.
8. Chilled Water System:
Acceptance Criteria: The chilled water system should provide water at the required temperature and flow rate to support cooling processes.
9. Hot Water System:
Acceptance Criteria: The hot water system should supply water at the specified temperature and pressure for various cleaning and sanitization activities.
10. Vacuum System:
Acceptance Criteria: The vacuum system should demonstrate the ability to achieve the required vacuum levels for processes such as drying, filtration, and distillation.
11. Cooling Towers:
Acceptance Criteria: Cooling towers should operate efficiently to dissipate heat from various processes and maintain cooling water at the specified temperature.
12. Temperature and Humidity Mapping of Storage Areas:
Acceptance Criteria: Storage areas should be mapped for temperature and humidity to ensure that pharmaceutical products are stored under appropriate conditions.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
What is operational qualification protocol (checklist) template?
You already know that it is a common practice to design both installation qualification and operational qualification tests in the same protocol.
That will provide you with an upper hand in merging the tests, troubleshooting, and final reporting of the verifications.
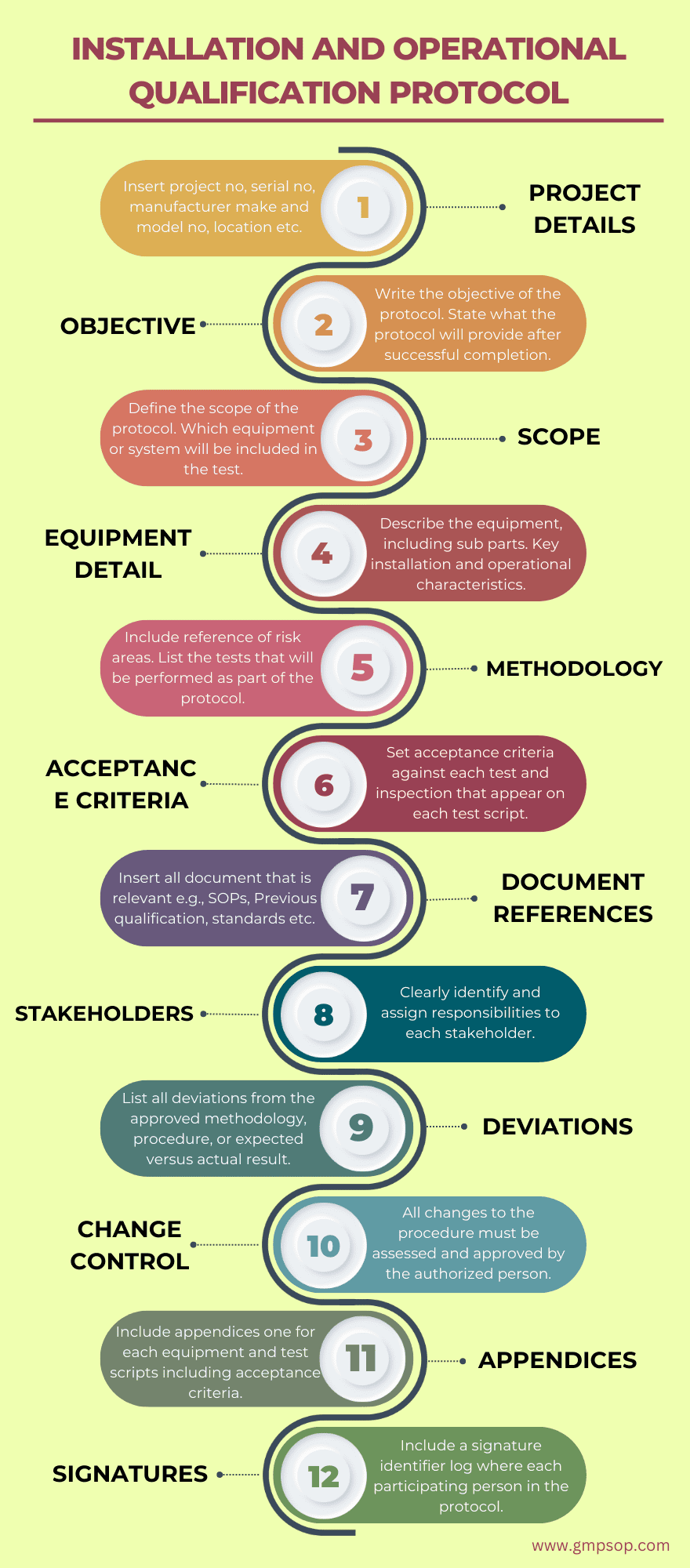
Following are the key components of an installation and operational qualification that you should use when building your protocol:
1. Include project details such as:
– Project name
– Project number
– Equipment
– Serial Number
– Manufacturer
– Model Number
– Process Line/Location
– Protocol number
2. Add the objective of the installation and operational qualification protocol
Write the protocol’s objective, which defines the installation (IQ) and operational qualification (OQ) requirements, as well as the acceptance criteria for the equipment and its location, such as packaging or production, and the facility.
Include a brief explanation of why certain criteria (IQ/OQ) are necessary, such as new equipment.
Provide a statement such as, “The successful completion of this protocol will provide a high degree of assurance that the equipment was installed in accordance with the site requirements, specifications, and manufacturer’s recommendations, and is in compliance with cGMP and site policies.”
Include the operational qualification objective as well, noting that with a successful OQ, you can check if the equipment is operating correctly and consistently within its operational limits.
3. Develop the scope of the operational qualification protocol.
Create the scope of the protocol by including equipment or system name. Include all sub-systems that are part of the main system. If there is a validation protocol provide a reference of protocol no.
Include the other equipment or system within the same area that are not part of the protocol. If applicable, reference the document number in which they are validated e.g., the cleaning validation number.
4. Provide a description of the equipment or system
Give a detailed description of the equipment and how it works. This should be kept to one or two paragraphs and should concentrate on the GMP as well as essential installation and operational aspects of the device.
A manual may provide the information, but it may need to be adapted to the protocol.
5. Provide operational qualification methodology to be used
State how the validation study will meet site and cGMP requirements.
Include the reference to the risk assessment that was conducted. Detail how would the qualification study will target the risk areas that are considered to pose a high risk to product safety, efficacy, purity, and identification.
Now list all the tests both installation and operational qualifications that will be performed as part of the protocol.
6. Develop the acceptance criteria against which the results will be measured
You must define acceptance criteria for each test that appears on each test script. When all of the following conditions are met, the device is regarded as qualified:
– All activities on the checklist have been completed.
– The installation and operation of the equipment or system have met the acceptance requirements for each test.
– All data entries have been signed and dated by the individual or persons who performed the work.
– All tests have been examined.
– Any deviations encountered during qualification have been satisfactorily rectified or accepted and approved.
7. Add references to the documents that are used to develop operational qualification protocol
Insert all document that is relevant e.g. SOPs, Previous qualification documents if this is a requalification, standards such as standards, etc.
8. Identify the stakeholder’s responsibility and authority during tests
Clearly identify and assign responsibilities to the Validation team, Engineering, Production, Quality Assurance, and Regulatory Compliance teams. Identify who has the independent authority to finally assess the results and approve the protocol.
9. Describe how the deviations will be handled during installation qualification?
During qualification studies, unplanned departures from the protocol are usual.
You should create a deviation log in which you record all deviations from the approved approach, procedure, or expected vs actual outcomes.
Keep records of full investigations, assessment summaries, and final corrective measures.
Demonstrate how those variations may or may not have influenced the overall outcome of the operational qualification study.
The installation and operational qualification cannot be deemed complete unless all critical deviations are corrected. Before the protocol can be completed, any deviations must be handled or justified.
10. Add a section for change control and re-validation
If you will experience a change to the protocol you have to deal with proper change management procedure. All changes to the procedure must be assessed by the authorized person and approved before the amendments can be made.
11. Include appendices in the protocol
Include appendices one for verification test scripts and acceptance criteria for each equipment, component, or system that are under test within the scope of the protocol.
In each appendix include the test number, equipment number and description, installation date, acceptance criteria, test results, test completed by name, and signature.
12. Signature identifier
Include a signature identifier log where each person who has participated in the installation qualification is identified by printed name, company/position, and corresponding signature and written initials.
How to perform operational qualification, step by step
Installation qualification is a process. You can break it into five different steps to complete one successfully.
1. Develop an IQ/OQ Protocol: create a detailed IQ/OQ protocol based on risk assessments, equipment specifications, operating parameters, tests, and acceptance criteria.
2. Pre-qualification Checks: conduct pre-qualification checks to ensure equipment installation and calibration are in order.
3. Perform Testing: Execute the IQ/OQ protocol by conducting various tests, such as functionality, accuracy, and safety checks. Refer to the previous section where we have listed a number of tests and corresponding acceptance criteria on commonly used critical and bespoke pharmaceutical equipment and systems.
4. Record Data: record all test data accurately and comprehensively during the OQ process.
5. Analyze Results: analyze the test results against predefined acceptance criteria to determine compliance.
6. Address deviations: If any deviations are identified during IQ/OQ, investigate and resolve them appropriately.
7. Compile IQ/OQ report: prepare a comprehensive OQ report, summarizing the validation activities, results, and conclusions.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
What is included in the operational qualification report?
After you complete the verifications, you have to compile all your findings and observations into an installation and operational qualification report.
The report should include:
– Introduction and objectives
– Equipment details and specifications
– IQ/OQ protocol summary
– Test results and acceptance criteria
– Deviations and corrective actions
– Conclusion and recommendation
Best Practices for successful operational qualification
Follow these best practices to ensure a successful operational qualification:
– Thorough planning
– Comprehensive risk assessment
– Robust OQ protocol development
– Accurate and thorough data recording
– Proactive deviation handling
– Collaboration and communication among the validation team
Conclusion
Operational qualification (OQ) is the second verification step after installation qualification.
The objective of OQ is to verify if the equipment is functioning correctly and consistently within its operational limits. It confirms the functional specification i.e., how the software will meet user needs, method to capture data, logic, and calculations, etc.
By following the step-by-step guide and adhering to best practices, you can achieve successful operational qualification, reduce risks, and ensure compliance with regulatory requirements for your business.
In this article, we have argued when you need to carry out an operational qualification based on the criticality of the equipment or system.
We have demonstrated how non-critical equipment is spared from OQ while commercial off-the-shelf (COTS), critical, and bespoke equipment and systems need various degrees of operational qualification testing.
We have created a strong rationale behind that qualification decision and also prepared references from the GAMP (Good Automated Manufacturing Practice) categories in support of the choices you can make.
We have managed to prepare a comprehensive list of OQ test examples including relevant acceptance criteria for commonly used critical pharmaceutical equipment and systems. We hope this will save you considerable time while developing an operational qualification protocol at work.
The development of installation and operational qualification protocol is of the utmost important task for your success. The essential elements of creating the IQ/OQ protocol that we have detailed in this document are the best industry practices.
Properly qualified equipment is critical for producing high-quality products and maintaining the overall efficiency of operations in regulated industries.
FAQs - operational qualification (OQ) in Pharmaceutical Industry
1. What is operational qualification (OQ)?
OQ confirms that the equipment is installed, calibrated, and capable to operate according to specification all the time.
It involves testing and documenting the equipment and systems to ensure they consistently perform within their specified operational limits and meet predefined acceptance criteria.
2. What is the purpose of operational qualification in validation?
The main purpose of operational qualification is to demonstrate that the equipment and systems are functioning correctly and capable of producing reliable and consistent results. OQ ensures that the equipment operates within acceptable parameters and adheres to regulatory requirements.
3. How does operational qualification differ from other validation tests?
Operational qualification (OQ) is one of the three main phases of equipment validation tests, alongside installation qualification (IQ) and performance Qualification (PQ).
IQ confirms that the equipment is properly installed, and OQ focuses on testing the equipment’s functionality within defined operational ranges.
PQ evaluates its performance under actual operating conditions.
4. What equipment and systems require operational qualification?
Almost all critical equipment and systems used in pharmaceutical manufacturing, such as autoclaves, tablet compression machines, HVAC systems, and laboratory instruments, require operational qualification to ensure product quality and patient safety.
Follow the GAMP categories to identify which equipment require what qualification testing.
5. What tests are performed during operational qualification?
Tests conducted during operational qualification vary based on the equipment or system being validated. Common tests include temperature uniformity, pressure, and flow rate verification, accuracy of measurements, alarm system functionality, etc.
6. What is included in the operational qualification protocol (checklist) template?
The operational qualification protocol (checklist) template includes a detailed outline of all tests and procedures to be performed during the OQ process. It outlines acceptance criteria, testing methods, equipment setup, and data recording requirements.
7. How to perform operational qualification, step by step?
Performing operational qualification involves carefully following the protocol and conducting each test as specified. This includes equipment setup, data collection, result analysis, and preparation of the final OQ report.
8. What is included in the operational qualification report?
The operational qualification report includes a summary of the OQ process, detailed test results, analysis of data, compliance with acceptance criteria, any deviations encountered, and a conclusion on the equipment’s performance.
9. What are the best practices for successful operational qualification?
Some best practices for successful operational qualification include thorough planning, clear documentation, adherence to protocols, proper training of operators, and proactive identification and resolution of issues.
10. Can Operational Qualification be combined with other validation phases?
While operational qualification is a distinct validation phase, it can be combined with other phases like installation qualification (IQ) and performance qualification (PQ) to streamline the overall validation process, especially when multiple systems are interconnected.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.
Related Posts
How to process packaging and labelling for clinical supplies
Six steps procedure for corrective and preventive action
Surface sampling swabbing method for microbiology tests