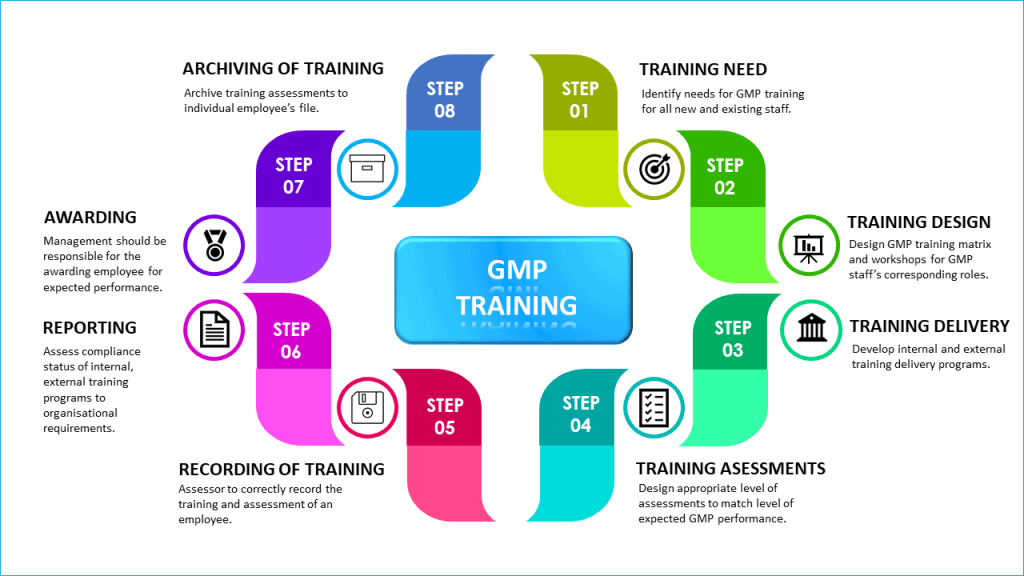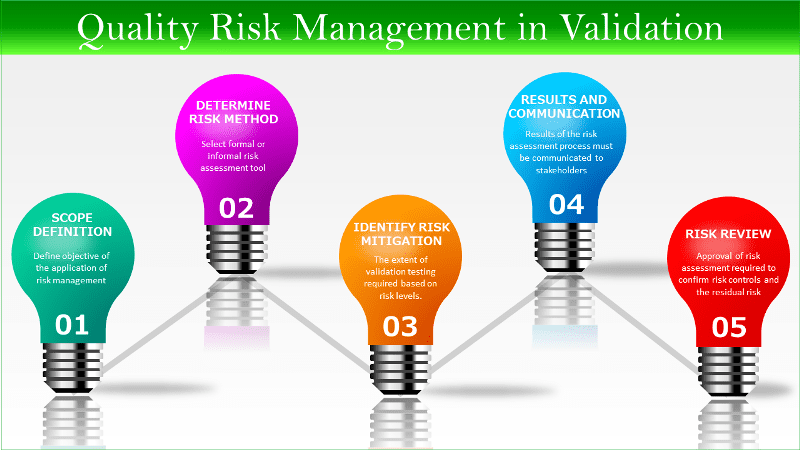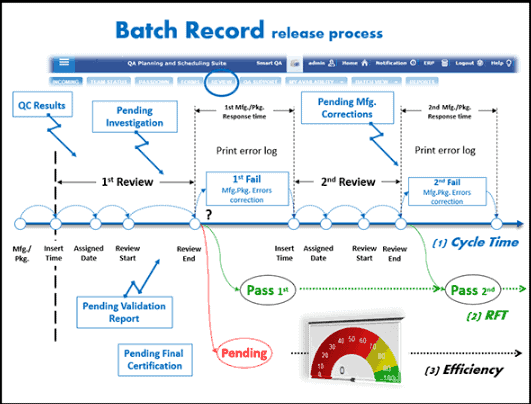
Access by Regulatory Authorities and Auditors to Electronic Records
- Published on: Oct 27, 2017
Requests for electronic records to be provided to an inspector from any regulatory authority, or other external auditing group, should be handled on the basis of a single set of principles as set out below. (This should apply equally to internal requests for copies so that the integrity of any copy can be assured.) The legitimacy of the request can be judged from the same rationale that would be applied if the record was paper-based and called for under any of the
GxP regulations or guidelines, or other regulators published requirements.In practical terms the formats of electronic records are often restricted by the system capability and where this is the case it should be made clear in the system documentation. In general it is good practice in advance of an inspection to seek advice regarding the formats of electronic records that might be required by or be acceptable to, an inspector so that this can be checked against capability and expectations can be managed.
Records provided as part of a formal submission to the FDA must be identified in public docket 92S-0251 as being the type of submission the agency accepts in electronic form. This docket will identify specifically what types of documents or parts of documents are acceptable for submission in electronic form Persons are expected to consult with the intended agency receiving unit for details on how (e.g. method of transmission, media, file formats, and technical protocols) and whether to proceed with the electronic submission.