
Equipment Cleaning Practices for Drug Products Manufacturing
- Published on: Oct 27, 2017
This article defines general cleaning requirements for facilities and equipment for Drug Products. This article applies to all GMP Production Sites where drug products are manufactured and/or packaged for Pharmaceutical and Animal Health.
Product Contact Equipment, both Major and Minor, used in production, Subpision, or sampling of a drug product, In-Process Material, or Raw Material (RM) shall be cleaned and shall include, and not be limited to:
- Changeover Cleaning;
- Interval Cleaning during a Campaign, as necessary; or
- Dedicated Equipment Cleaning at the end of a campaign. Equipment disassembly may be required to clean or to Verify cleanliness.
Equipment Cleaning for major equipment must be conducted following written Instruction-Records or Standard Operating Procedures (SOP) with an attached checklist(s). These documents shall be Approved by the Site Production Team and Site Quality Team before being issued.
Equipment cleaning for minor equipment shall be conducted following written SOPs or Instructions-Records and these cleaning activities must be documented. The SOPs and Instruction-Records shall be approved by the Site Production Team and the Site Quality Team before being issued.
Executed Instruction-Records or checklist used shall be reviewed and approved by the Site Quality Team. Use and Cleaning History must be determined for product contact equipment that has been used by third parties or by another facility (e.g., trials, rentals, borrowed). The history must be documented and approved by the Site Quality Team. The removal of previous product residues must be verified, prior to any use.
The history shall demonstrate that the equipment was not used for and does not contain potential contamination from objectionable materials (e.g., penicillins or other beta lactams, pesticides).
Equipment Cleaning shall be designed to prevent Cross Contamination including microbiological contamination, when applicable [based on the Risk Assessment, by reducing residues on all product contact surfaces to acceptable levels. Maximum Allowable Residue (MAR) and Residue Acceptability Limit (RAL) for Equipment Cleaning shall be established by Qualified personnel based on the empirical data and the approved calculation method, prior to any use.
Swab or Rinsate Sampling Methods must be Validated through use of recovery studies. The Analytical Methods used to test for residue must also be validated. For Changeover Cleaning, Routine Verification of Cleaning Processes for Major Equipment Where One Hundred (100) Percent Visual Inspection is not Possible shall include:
- Inspection to the extent practical to verify the equipment is Visibly Clean;
- Periodic monitoring (e.g., using swab or rinsate sampling method as per validation) at a frequency defined based on a documented and approved risk assessment (e.g., up to 2 to 3 years) of the probability of contamination; and
- Approved justification of the rationale for the frequency of the periodic monitoring.
Routine Verification of Cleaning Processes for Major Equipment Where One Hundred (100) Percent Visual Inspection is Possible shall include inspection to verify the equipment is visibly clean (e.g., mills, filter housings, and tray driers).
Lighting shall be sufficient to facilitate visual inspection for residues. For areas to be visually inspected, the equipment must be dry before inspection. Routine Verification of Cleaning Processes, for minor equipment shall include visual inspection to verify the equipment is visibly clean.
Lighting shall be sufficient to facilitate visual inspection for residues. For areas to be visually inspected, the equipment must be dry before inspection.
If the Only Verification of Cleaning Processes to be Conducted on a Piece of Equipment is Visual, then the visually detectable quantity must be known and documented.
A Qualified Inpidual other than the one who performed the cleaning must verify that the equipment is visibly clean.
Commercial Production Equipment, for a one time product (e.g., clinical Batches/Lots), for which cleaning has not been previously validated, must be verified as clean by visual and residue testing after the production of the product, before the next use of the equipment. The Need for Cleaning, Type or Level of Cleaning, and Cleaning Frequency within a Campaign shall be defined, and documented.
Interval cleaning shall be performed as required during a campaign. Product Changeover Cleaning Procedures must be validated for all product contact equipment used for multi-product production and sampling of drug products.
Personnel Responsible for Equipment Cleaning Operations and Sampling must be trained and qualified in these operations. Such training, along with Evidence of Training Effectiveness, shall be documented.
Approved Cleaning and Sanitizing Agents shall be used to clean product contact equipment and shall be compatible with the surfaces to be cleaned. These agents shall be purchased, received, stored, approved, dispensed, and transferred similarly to raw materials.
Equipment Cleaning, Use and Maintenance Logs, consisting of chronological entries on sequentially numbered pages or equivalent validated electronic System, shall be maintained for each major Equipment Unit, and for each major Equipment Item that is not included in a defined equipment unit. Information to be recorded shall include, and not be limited to, the following:
- Documentation of cleaning, use, and maintenance;
- Date of the activity;
- Start and finish times of the activity;
- Equipment use, including product name and Batch Numbers or Lot Numbers of each lot or batch processed in the equipment;
- Signature or initials of inpidual performing the work; and
- Signature or initials of a second person verifying the work, where no other primary Records are in place to document the work.
For non-electronic based equipment cleaning records, use and maintenance logs, the issuance, revision, retention and reconciliation of pages must follow Site procedural control as per primary records, such as batch records.
For Each Equipment Unit or Item, the Cleanliness Status shall be indicated using labels and/or signs or a centralized paper-based or validated electronic system. Cleaning status can only be changed to CLEANED once all monitoring results (when applicable) are obtained and found to be satisfactory. Examples of equipment cleaning status include, but are not limited to:
- Hold for Cleaning,
- Cleaning, and
- Clean.
Measures to Protect Clean Equipment must be taken during storage to prevent environmental or microbial contamination. For example:
- Store equipment dry,
- Close tank openings,
- Close tablet machine covers,
- Cap or cover open flanges, and
- Protect portable equipment (major and minor) from environmental exposure by sealing in a protective bag or by shrouding and storage in a clean location. Cleaned Portable Equipment must be stored, segregated from, in-use and/or unclean equipment.
Pre-Use Inspections shall be performed immediately prior to use following a changeover cleaning or dedicated equipment campaign cleaning, and documented (e.g., in the next batch or campaign record). Pre-use inspection shall verify that the equipment:
- Has been properly assembled (e.g., via visual inspection or by communication with the responsible personnel);
- Has been cleaned;
- Is visibly clean;
- All identification from the prior product has been removed;
- Cleaning records are verified for completion;
- Analytical results (if applicable) are satisfactory; and
- Maximum allowable time period between cleaning and next use was not exceeded (if applicable).
Maximum Allowable Time Intervals for Periods Between Use and Initiation of Cleaning shall be evaluated. If this time period potentially impacts the ability to clean the equipment then the time period must be specified and validated.
If the maximum allowable time intervals are exceeded, the equipment must be re-cleaned and inspected prior to use. If applicable, the equipment shall also be sampled (e.g., for potential microbiological contamination). The re-cleaning and cleaning verification shall be documented (e.g., logbook, cleaning instructions or checklist).
After Completion of any Maintenance or Instrument Calibration Activities that required opening or disassembly of equipment, an evaluation must be conducted and documented to determine the level of cleaning required, if any, for product contact equipment.
Prior to Being Put into Service, New Equipment shall be cleaned and, the equipment shall be verified as visually clean at a minimum. The cleaning and verification of cleanliness must be documented and approved prior to use.
Equipment Cleaning Failures During Routine Monitoring (i.e., visual and/or analytical result failures) must be documented and Investigated according to established Site procedures. An evaluation of the impact on validation must be included.





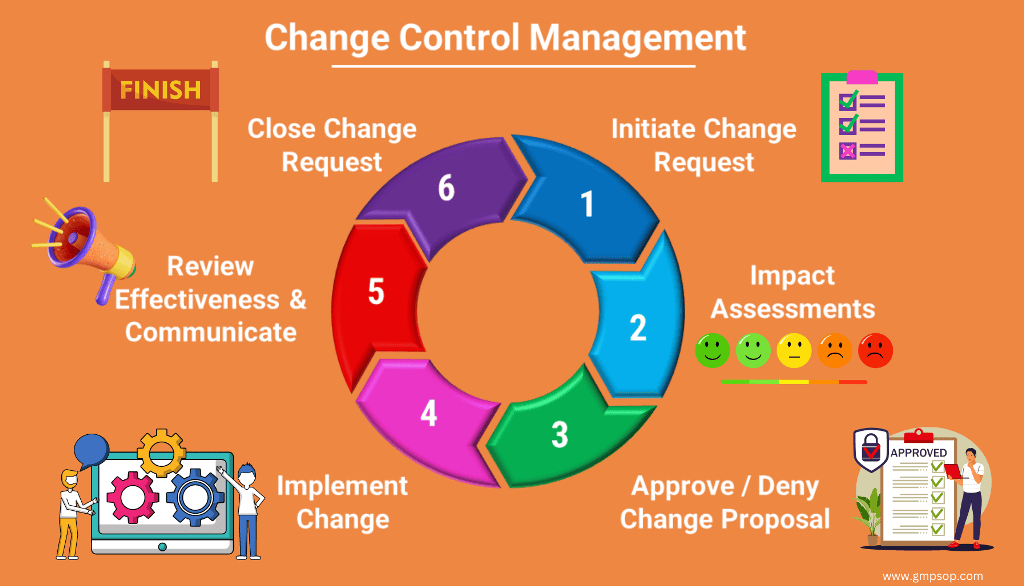
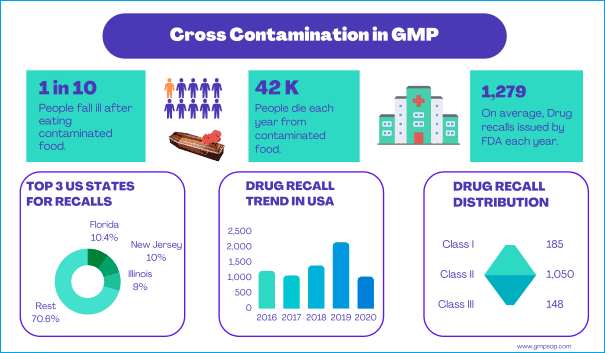
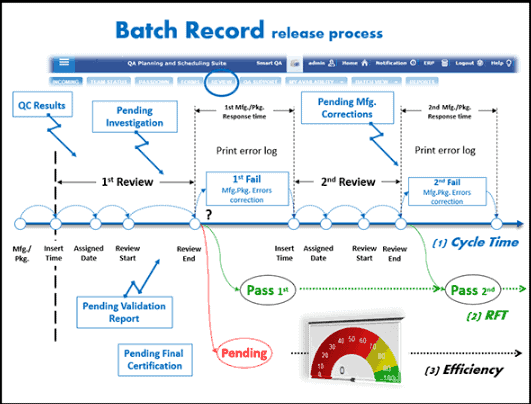
Intervals of cleaning for unused equipment example if I do not have a production plan for liquid line for 10 days and the equipment was cleaned after the last batch can I leave the tanks without cleaning till the time of use
Can you please tell me which type of minor cleanning could be used for ointments and creams production ?