Guidelines for Regulatory Inspections at a GMP Site
- Published on: Oct 27, 2017
It is essential that management develop, document and implement procedure(s) for managing inspections by Regulatory Authorities in order to protect the legal rights of the business (and the Regulatory Authorities to perform repeated inspections) and at the same time, to maintain a professional relationship with the regulatory authority conducting the inspection.
Senior Management at a site or function must be present during key parts of an inspection.
Inspection notification, ongoing highlights of the inspection, and the final results of an inspection must be communicated to relevant Senior Management in a timely manner.
There must be a local SOP describing the actions and responsibilities associated with an inspection. The SOP must address the local legal requirements for taking photographs; the use of tape recorders or other electronic equipment; the listening to, reading and signing of affidavits by company personnel; the review of internal audit reports and allowing access to computer databases.
An accurate and detailed record is to be maintained of events, significant comments or recommendations from the inspector(s), documents and / or reviewed/copied for the inspector, product samples, and any other information deemed important to the inspection.
At the conclusion of an inspection, local management must assure that any inspection observations are clearly understood, that any factual errors are brought to the attention of the inspector(s) and that there is a clear understanding of what will be addressed by the site in written communication to the Authority.
Following a detail procedure that can be followed by any GMP site for preparation of inspection by regulatory authority.
The purpose of the procedure is to define the procedure for the conduct of GMP sites employees involved in regulatory authority inspections. This procedure shall be used to guide GMP site personnel in contact or involved with the assistance of any external third party regulatory authority inspection.
It is the responsibility of the Management Representative to implement and maintain this procedure.
Preliminary meeting:
The Inspector(s) and the responsible person are required to attend the preliminary meeting. Representatives from the following departments may be requested to attend the preliminary meeting if required:
- Regulatory Affairs
- Human Resources
- Customer Service
- Service and Repair
- Warehouse
- Finance
- Sales and Marketing
- Site layout
- Site activities
- Normal hours of work
- Scope, duration and timing of the inspection
- Standards/Regulations which the inspection will be assessed to be compliant
- Key personnel required to be available
- Schedule for inspection, daily and final review meetings
- Ensure the inspector is accompanied during the walk-through of the facility
- Act as a primary source of information
- Ensuring, where appropriate, that questions are directed to designated personnel
- Keep a list of all samples and copies of all documents given, and marked “Confidential”, if appropriate
- Keep notes of any Nonconformities or observations identified by the inspector and begin corrective action(s) immediately (where appropriate) with the relevant Department Manager
- Courteous and cooperate at all times
- Make sure the inspector’s questions are clearly understood, not attempting to anticipate questions
- Answer the question being asked and not volunteer information
- Not let the inspector browse through files, only present the requested document(s)
- Answer questions truthfully; if a question cannot be answered, or the answer is unknown, this should be made clear
- Maintain a written record of all the inspector’s observations, comments and requests for samples, documents, etc., for files and reference.
- At the end of each day, a review meeting may be held involving the inspectors and key personnel participating in the inspection at which:
- The inspectors will be asked to detail any apparent non-conformance observed during the day
- The inspector’s findings will be reviewed and the opportunity taken to correct any misunderstanding
- The inspectors will be asked to detail any apparent non-conformance observed during the inspection
- The inspector’s findings will be reviewed and the opportunity taken to correct any misunderstanding
- Give a verbal response, either immediately or after retiring to consider the matter
- Reserve the position until review of the inspector’s written report.
- Once the final inspection report is received from the responsible auditor, site quality authority shall provide a written response addressing all findings with an action plan, time frame and responsibility for action. The response to the final inspection report shall be approved by the responsible person(s).
- The response to the final inspection report shall be sent to the responsible auditor within the time frame provided in the final inspection report.
- As findings are addressed, corrective actions are implemented and the effectiveness of the actions are verified, these shall be communicated to the designated contact responsible for the inspection report.
- The inspection will not be considered closed until all findings are addressed, corrective actions are implemented, and the actions closed out by the regulatory authority inspector.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.
Related Posts
You may like to read these related posts.
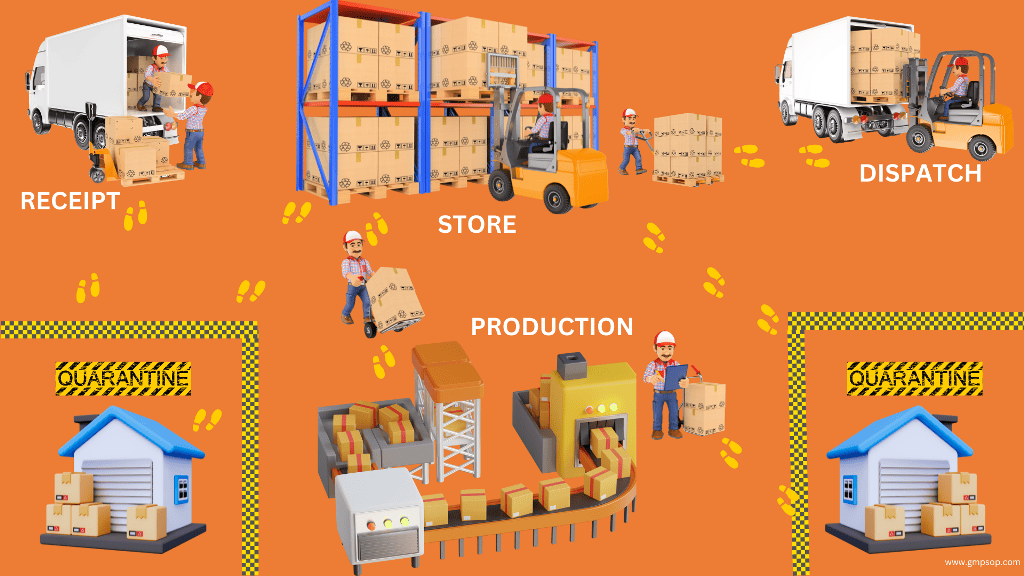
Warehouse material handling for pharmaceutical industry
Quality Assessment for Reworking Active Pharmaceutical Ingredients and Drug Products
Line clearance procedure and reconciliation in GMP