Preventative Measurements for Eliminating Cross Contamination During Medicinal Product Preparation
- Published on: Oct 27, 2017
The ultimate objective of a good quality management systems and practices within a GMP facility is to make sure the medicinal products we manufacture and distribute are safe, pure & effective for human use and traceable, as such, if a post marketing quality issue is discovered, proper investigation can be performed.
While the product efficacy is ensured during the product development and clinical trial phases, the safety purity and traceability are established at the time of products are manufactured, tested and released for distribution.
Preventing the medicinal products from external contamination is absolutely critical. Evaluating the complete manufacturing process flow should be done to identify and eliminate external contamination sources. Critical control points are set to prevent any possibility of contamination during each phase of the drug product preparation. The preventative control measures start from the point of sourcing the medicinal actives, excipients and packaging, testing as per specification and releasing those for production. Controls are put in place during the production processes, pre and post production storage facilities and conditions, material transfer and distribution.
The GMP facility should be designed to include the considerations of room and equipment layouts to minimize potential cross contamination of products (e.g. use of airlocks). Use of closed processing equipments, air quality, water quality, airflow requirements and air recirculation controls help the product from being exposed to external environments. Controls should be implemented for product and material flow, personnel flow and gowning requirements appropriate to environmental grades. Procedures to be created for collection, transportation and storage of process waste, drainage systems, cleanable continuous smooth and non-porous floors, walls, doors and ceilings with surfaces able to withstand repeated application of cleaning agents.
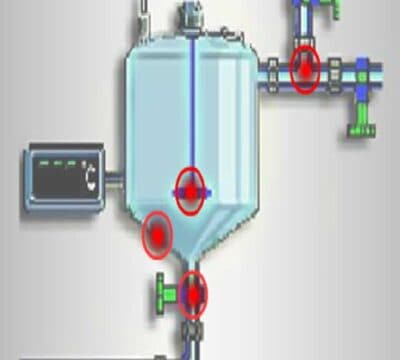
Precautions should be established and maintained to ensure that cross contamination at product exposure points (e.g., open hatch charging or sampling, packout, heel scraping) is prevented from overhead equipment and piping.
Consideration should be given to such precautions, including the booth or canopy over tank hatch openings, isolators (e.g. glove boxes), SOPs defining conditions under which vessels can be opened, periodic inspection of overhead piping and ducts to ensure that there are no leaks, condensation, flaking of paint and/or insulation that might fall into vessel openings, drip pans under HVAC units or cold surfaces to collect or contain condensation and facilities designed with minimal overhead ducts and piping.
Equipment should be located so that cross contamination between production operations undertaken in a common area is prevented.
Ovens, dryers, autoclaves and similar equipment should contain only one open, exposed raw materials, active pharmaceutical ingredients, intermediate, in-process material, medical device or drug product at a time.
Raw material sampling should be performed in an area dedicated to sampling (e.g., sampling booth).
Where dust is generated (e.g., during sampling, weighing, mixing and processing operations, packaging of dry products), dust collection or containment systems should be used to avoid cross contamination and to facilitate cleaning. dust collectors of any type should not exhaust into any storage or production areas.
Clearly defined manufacturing steps, facility and equipment design, process layouts and proper execution of standard procedures are vital to keep the medicinal products away from potential cross contaminations. Good cleaning practices, validated testing regime and real time documented evidence can produce assurance that the manufactured drug products are free from contaminations and safe for human consumptions.