Evaluation of Contaminant Options for Packing of Solid Dosage Forms
- Published on: Oct 27, 2017
Company product portfolio may includes a wide range of active materials. Effective use of those actives requires a clear recognition and understanding of the hazards, how to manage the associated risks and adequately control employee and patient exposure.
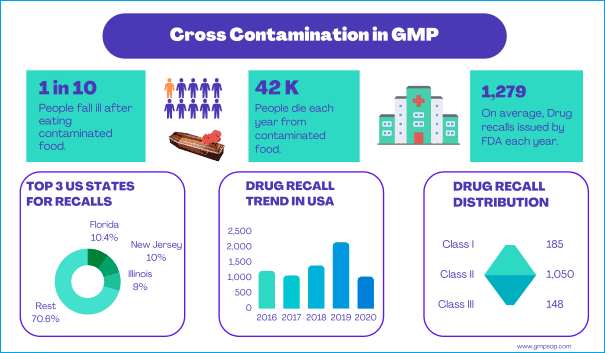
1. Experience has shown that historically there have been significantly different approaches taken to the management of containment and cross contamination reduction.
2. Uncertainty and lack of clarity regarding requirements can result in inconsistent application of design and operation standards.
3. A number of common questions affecting accommodation/control decisions have arisen from different areas of the Company. This has resulted in duplication of effort and the potential for application of inconsistent solutions.
4. Decisions affecting packing operations have sometimes been based on hazard or perceived hazard, rather than risk. A specific example is products in the class (cytotoxic). Whilst all products in this class (kill cells), the mechanism of action and specific properties of individual products in this class may be different, and may require different solutions to accommodate them.
5. Decisions affecting packing operations have sometimes been taken based on an inconsistent interpretation of GMP guidelines.
Key factors affecting the design and operation of packing facilities and equipment (existing or new) should include:
- Operator protection
- Environmental protection
- Protection of patients in the community
- Product associated hazard and risk
- Company standards
- Engineering standards
Packing engineering technology options: A project team with representatives from Manufacturing Strategy Group, Safety management, Engineering, GMP Compliance should be set up to identify the key issues and how to address them.
The main issues affecting the company are:
- For a multi product company, aims should be to build flexible multi-product facilities.
- Need to provide and use Company assets in a cost-effective way whilst maintaining the license to operate.
- Different designs solutions carry different costs.
- Design decisions do not always take a rational and consistent view of regulatory issues (health/hygiene/GMP).
- Lack of clarity around the relative importance of hygiene, GMP and engineering issues leading to inconsistent/inappropriate design and operational decisions.
- Decisions have sometimes been made on the basis of hazard, or perceived hazard, rather than risk.
Related Posts
What is analytical method validation in GMP
Microbiology and Good Laboratory Practice in GMP
How to effectively control packaging materials in pharmaceutical