Deviation Investigation Guidelines in GMP Facilities
- Published on: Oct 27, 2017
Deviation investigation is a mandated requirement for pharmaceutical production process. Deviations to approved Production, Testing, or Distribution Procedures for Active Pharmaceutical Ingredients (API), Drug Products, Medical Devices, Consumer Health Care (CHC), Animal Health, and cosmetic products produced by a GMP site or confirmed Out-Of-Specification (OOS) Results obtained during testing of such products shall be documented, Investigated and Disposition to be made.
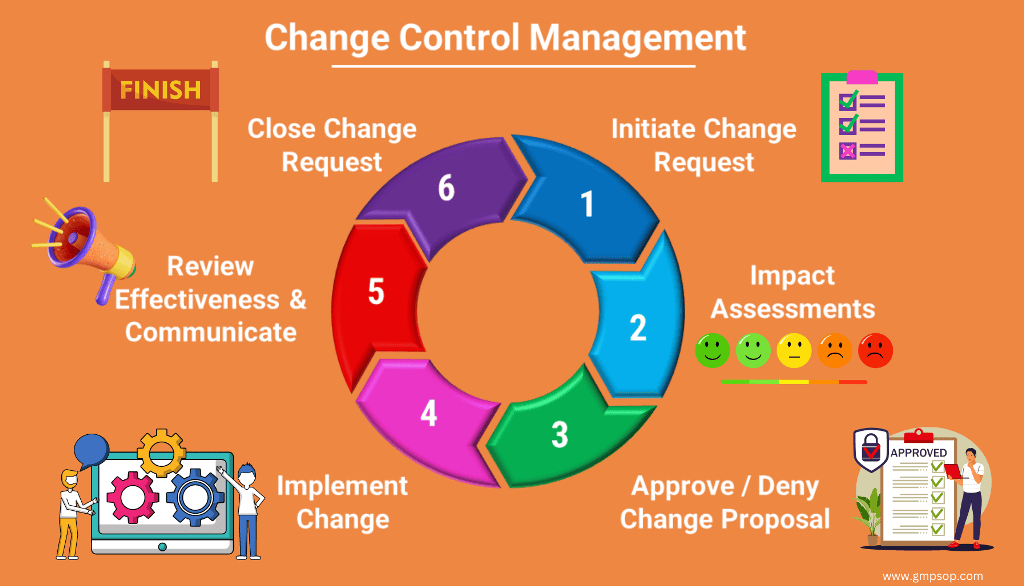
Planned temporary deviations shall be approved prior to initiation of the deviation. Planned permanent changes shall be addressed by the change management system.
Each colleague is responsible for identifying deviations and reporting incidents to department supervision.
The Site Quality Team shall be notified of all deviation investigations.
Planned Temporary or Unplanned Deviations shall be documented in a Deviation Report (DR).
The Supervisor responsible for the department where the Deviation occurred shall document the results of the deviation investigation in the Deviation Report, review the deviation report and forward the report to the Site Quality Team. Either the deviation report form or a site-specific form that meets the requirements of this procedure shall be used.
– The deviation report shall be approved by the Site Quality Team.
– The deviation reportshall:
- Be assigned a unique number;
- Document any decision regarding the need for any Market Action for a Batch/Lot already approved anddistributed
- Contain a final statement regarding product quality;
- Contain a conclusion regarding the probable disposition (Approved, Rejected, or Reprocessed) for a lot/batchthat has not been assign final disposition; and
- Be tracked and Trended by the Site Quality Team.
210 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
The Site Quality Team shall evaluate deviations (planned temporary and unplanned) and assess the potential impact to product quality.
Final Disposition of a Batch/Lot shall not be assigned until the deviation report has received final approval.
Responsibility for Batch Disposition is vested with the Site Quality Team.
All Unplanned Deviations shall be investigated by the Supervisor of the department where the deviation occurred and the Site Quality Team, with assistance from other experts, as needed.
The Site Quality Team shall call for an expanded investigation through the establishment of a Cross-Functional Team (CFT) using Root Cause Methodology, when one of the following situations occur:
- The cause of the deviation cannot be determined by the Supervisor of the department where the deviationoccurred;
- There is probable cause based on available evidence, but the cause is not confirmed, the establishment of a CFT is at the discretion of the Site Quality Team; or
- A deviation demonstrates repeated trends, or when repeated complaints result from a deviation.
Where an assignable cause cannot be determined by the CFT, the investigation shall be referred to the Site Quality Review Team or Senior Management Team for review and assessment.
The deviations that need specific attentions include, but are not limited to:
- Issues that could potentially result in NDA-Field Alert Reports
- Confirmed OOS result and Questionable Results related to stability or testing performed on Complaint investigations for marketed products;
- Specific lot or products;
- Deviations from regulatory filing and/or regulatory commitment on marketed lots or lots intended to be marketed;
- Labeling errors on finished marketed product;
- All potential or actual market actions;
- Regulatory inspection observations and commitments;
- Issues related to Expiration Dating of API, drug product, consumer product, medical devices, Biologics, RawMaterials or Components;
- Deviations from site Policies and Practices; and
- The Site Quality Team shall track deviation report corrective and preventive actions until completion and shall use the data for trending purposes.
Planned Temporary Deviations shall be documented, justified, and assessed for impact on product quality,
Validation and/or regulatory commitments by the initiating Department Supervisor and approved by the Site Quality Team prior to implementation.
Planned temporary deviation to a medical device must be reviewed, Verified and/or Validated as necessary and approved, prior to implementation.
Repetitive Planned Temporary Deviations shall be minimized and permanent changes shall be considered and initiated.
When an Unplanned Deviation Occurs or is Discovered, colleagues shall immediately notify the Department Supervisor.
All deviation reports shall be initiated by the Department Supervisor and submitted to the Site Quality Team for assessment within one (1) Business Day from the time of the discovery of the deviation.
In the Event of an Unplanned Deviation investigation, the Site Quality Team shall assess the potential impact on product quality within two (2) business days from the time of receipt of the deviation report.
The assessment shall be performed by the Department Supervisor where the deviation occurred, the Site Quality Team, and other experts as needed. The preliminary assessment shall include, at least:
- Potential quality impact,
- Regulatory commitment impact,
- Verification and/or validation impact,
- Other lots potentially affected, and
- Alert Reports.
- The deviation report for an Unplanned Deviation investigation shall include the following information:
- Deviation report number (as part of a Site document management and DR tracking system);
- Identification of the material or product description involved, including lot and code numbers, or system involved;
- Identification of master production or control document(s) involved and their current effective dates;
- Description, date, and time of the event or occurrence;
- Investigation details, including review of relevant quality systems, test data (when applicable), control charts (where applicable), and all lot(s) potentially affected
Description of the cause:
- Assignable;
- Probable with supporting evidence but unconfirmed; or
- Cause is undetermined, and the conclusion is summarized without speculation as to cause;
- Identification of whether the cause is a Common Cause or a Special Cause, if known;
The deviation report for an Unplanned Deviation shall include:
- Supporting rationale(s) for cause, quality impact, immediate corrective action(s), and preventive measure(s);
- Investigative conclusions regarding the batch/lot or system, immediate and/or long term corrective actionsand/or preventive measures with assigned responsibility and target completion dates;
- Approval signatures by, at least, the Manager of the department where the deviation occurred; and
- Final approval by the Site Quality Team.
The deviation report for a Planned Temporary Deviation shall include the following information:
- Deviation report number (as part of a Site document management and DR tracking system);
- Identification of the material or product description involved, including lot and code numbers, or system involved;
- Rationale justifying the deviation plan and an assessment of impact to product quality and validated state;
- Approval signatures by, at least, the Manager in the department where the planned deviation is to occur; and
- Final approval of the rationale and the plan including any follow-up, by the Site Quality Team.
- All planned deviation investigations must be approved prior to implementation.
Deviation reports for Unplanned Deviations shall be completed and given final approval within thirty (30) calendar days from when the deviation is first reported to supervision. When final approval for a DR cannot be completed by that time, an interim DR will be completed within thirty (30) calendar days of when the deviation is first reported to supervision, and every thirty (30) calendar days thereafter until the final DR has been given final approval.
If a deviation report is not completed and been given final approval within ninety (90) calendar days from the date the deviation is first reported to supervision, the Site Quality Team forwards the interim deviation report to the Senior Management Team (SMT).
The Interim deviation report shall include the current status of the investigation, the reason for delay in the completion, and the expected date of completion.
The Site Quality Team shall be responsible for approving all final and interim deviation report.
For Deviations brought to the SMT, the SMT shall review the submission prepared by the Site Quality Team summarizing the elements of the deviation report and then either concurs with the Site Principals recommendation or asks for more data or work to be completed.
The SMT determines if regulatory action is required prior to release of the lot or if field action needs to be considered by a Market Action Committee.
Decisions by the Site Quality Team to reject a lot/batch cannot be overridden by the SMT.
Unusual Testing Results can indicate that a deviation exists. For example, if test results:
- Involve an OOS result or questionable result that is not attributable to a laboratory originated assignable cause, a deviation report is also required; in such an event, a copy of the relevant Laboratory Investigation Report (LIR) shall be attached or cross-referenced to the deviation report and information already in the LIR does not need to be repeated in the deviation report;
- Are outside quality action criteria but within regulatory limits, a lot/batch may be recommended for approval or reprocessed following review of the deviation report and concurrence with the Site Quality Team; or
- Are outside Alert Levels but within Action Levels, no action is required, other than to be aware that a potential deviation may be developing.
The Original deviation report shall be filed in the batch/lot Record when an API or drug product is involved and in the Device History Record (DHR) when a medical device is involved. Alternatively, the assigned deviation report number shall be referenced in the respective batch/lot record or DHR when the original report is filed in a central file system administered by the Site Quality Team.
When deviation report is not batch/lot related it shall be filed in the specific product/subject file.
The site system used to track deviation reports shall include all identified corrective and preventive actions.
When All Corrective and Preventive Actions are Complete, the Site Quality Team shall perform the final close-out of the DR by confirming that all actions were completed and documented.
The Site Quality Team shall trend deviation report by deviation category, product, and/or root cause at least annually. The trends shall be reviewed by the SMT, when there is a trend identified, a corrective action shall be put in place.
When an Electronic System is used at the site to track Corrective Actions, the system shall track the close-out of the corrective action and the tracking number shall be included on the original DR. It is then not necessary for the Site Quality Team to document final close-out on the original DR.
For a Drug Product or API manufactured outside the European Community (EC) or Canada and imported into the EC or Canada, copies of all relevant deviation reports, with quality or Regulatory Impact, must be forwarded by the sending Site Quality Team to the receiving Site Quality Team (who is a Qualified Person in the EC). Deviations concerning quality or regulatory issues must be approved by the receiving Site Quality Team prior to release to the EC or Canada.
Related Posts
How to Develop Quality Control Method and Specifications for a Laboratory
Quality Assessment for Reworking Active Pharmaceutical Ingredients and Drug Products
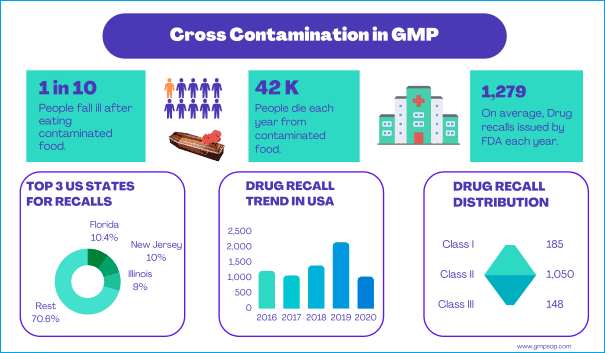
What is cross contamination in pharmaceutical industry