How to Manage Pharmaceutical Product Recall and Adverse Events
- Kazi
- Last modified: April 30, 2025
Pharmaceutical product recalls may result from severe adverse events directly attributed to manufactured products. Recalls can originate from adulterated products, serious complaints, or critical regulatory findings.
Medicinal products are highly regulated to prevent adverse events and ensure patient safety. Despite the regulations and robust quality assurance system, substandard products can slip through the process and end up in patients’ hands, resulting in adverse events.
This article explores the mechanism of managing pharmaceutical product recalls, reporting adverse events, and effectively executing market actions, ensuring compliance with local and international regulatory expectations.
We will explore the following areas:
– The reporting of patient incidents, adverse events, safety alerts, customer information bulletins, and product recalls.
– Communicate such actions and report when you need to.
– The notification to regulatory bodies by the regulatory affairs team.
– Raising and distributing advisory notices, safety alerts, and customer information bulletins.
– Approval, implementation, and reporting of product recall actions.
– Developing product recall through effective communication with the regulatory authorities.
– Conducting mock recalls to test your readiness.
– Developing and approving a market action strategy with regulatory oversight encompassing broader interventions beyond recalls, like field corrections and withdrawals.
Table of Contents
Key Takeaways
- Product recalls for Pharmaceutical products may result from severe adverse events or critical regulatory findings.
- Use multiple channels to detect and assess adverse events quickly.
- Follow a structured decision tree to determine if an event must be reported.
- Regulatory affairs (RA) along with the quality assurance (QA) must coordinate with manufacturers to submit accurate and timely reports.
- Safety alerts may be instigated by the manufacturer or upon the recommendation or request of a regulatory body.
- A customer information bulletin (CIB) is typically a document containing information similar to that within a safety alert but of lower significance.
- A multidisciplinary team should manage recalls under a recall coordinator.
- Maintain effective communication with both regulators and customers throughout.
- Market action encompasses broader interventions beyond recalls, like field corrections and withdrawals.
- Develop and approve a market action strategy with regulatory oversight.
- Ensure proper documentation, effectiveness checks, and final reporting to close actions.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Understanding Adverse Events and When to Report Them
An adverse event in the context of pharmaceutical or medical device use refers to any undesirable experience associated with using a product.
Reporting such incidents immediately is required by regulatory agencies worldwide as part of post-market surveillance.
Incident Reporting Channels
You must raise an initial notification when you become aware of a patient incident. You can use different communication channels to collect information about such incidents. These can be:
– Hospital or healthcare provider reports
– Manufacturer notifications
– Media coverage
– Product specialists or field teams
– Direct regulatory body alerts
These events must be documented using a product complaint and incident reporting form and assessed immediately by the regulatory affairs team.
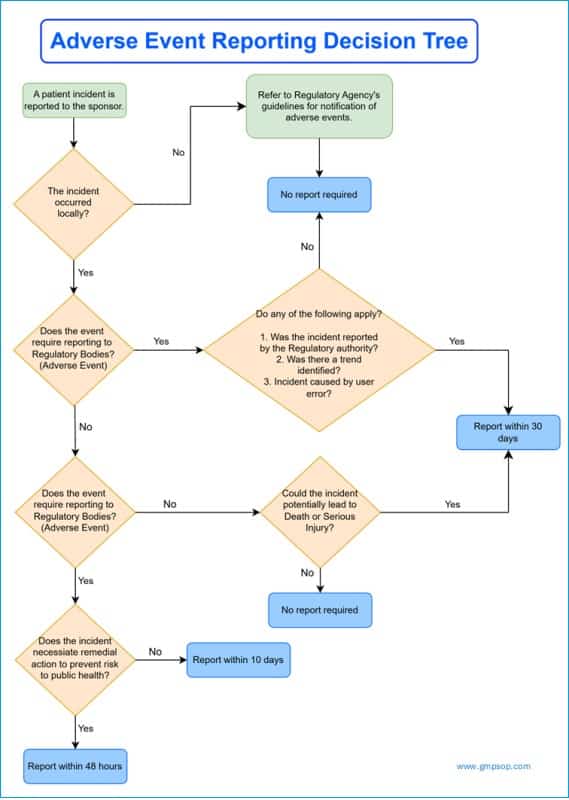
Risk-Based Assessment and Decision Tree
Using a structured decision tree, the regulatory team evaluates the incident’s severity and determines whether it requires reporting. The timing of the report submission is governed by the criticality of the event and applicable regulatory guidelines.
If the event is not deemed reportable, the rationale must be recorded in accordance with post-market surveillance practices. For pharmaceutical products, follow the guidelines from the FDA Recommendations on recalls. In the event of medical device recalls, you must follow the guidelines outlined in ISO 13485:2016.
How to Submit Adverse Event Reports to Regulatory Authorities
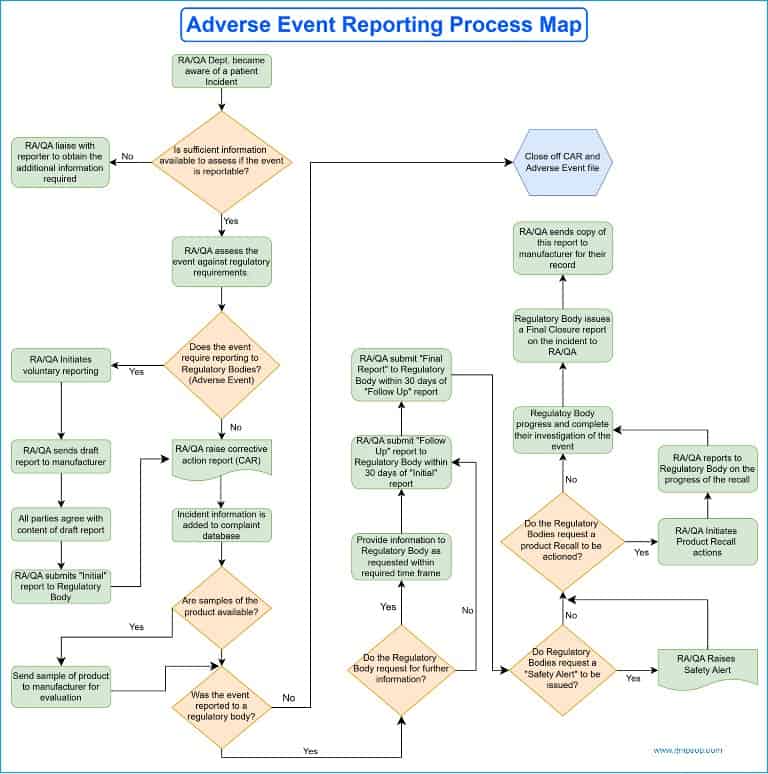
Where the patient incident is deemed reportable (e.g., an adverse event), the regulatory affairs team must enter all relevant details as required by the regulatory bodies and submit them with proper acknowledgment.
The legal manufacturer’s regulatory affairs team should review and amend any information in the report if required.
The legal manufacturer will typically be required to provide information about the number of devices manufactured and supplied to the market, as well as the number of similar incidents involving the product in both local and international markets.
When the draft report has been agreed upon with the manufacturer, the regulatory affairs team submits the initial report to the regulatory agency. In addition, the manufacturer is to be provided with a copy of the initial investigation report.
If a communication or request for further information is received from a regulatory body, it must be forwarded immediately to the relevant legal manufacturer.
All responses must originate from or be vetted by the manufacturer before submission to the regulatory agency. Subsequent communication must also be processed in the same manner.
Evaluation of samples
If a sample of the products or devices involved in the adverse event is available for evaluation, the regulatory affairs team should send it to the manufacturer.
Any samples being sent for evaluation must be processed to ensure they have been adequately decontaminated and certified.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
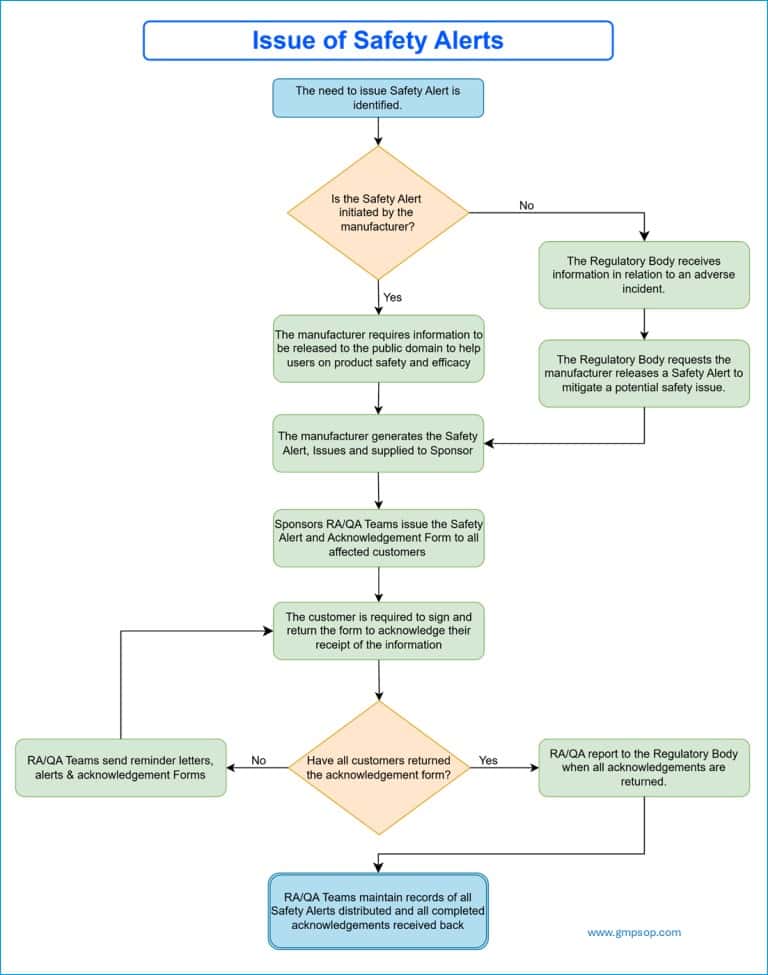
Issuing Safety Alerts for adverse events
Safety alerts may be instigated by the manufacturer or upon the recommendation or request of a regulatory body.
A safety alert issued by the manufacturer is information released to the public domain that will help device users use the device safely or more effectively.
Safety alerts requested by regulatory bodies may result from receiving information about an adverse incident in which they feel the manufacturer should make a public announcement to mitigate a potential safety issue.
Every safety alert issued to customers includes an acknowledgment form. The customer must sign and return the form to acknowledge receipt of the information.
You must retain all distributed alert records and all completed acknowledgment forms received.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Issuing Customer Information Bulletins
A customer information bulletin (CIB) is typically a document containing information similar to that within a safety alert but of lower significance in its ability to negatively affect the user’s health, safety, or well-being.
The information in a CIB is typically related to a particular operating characteristic, labeling, and operating instruction.
The CIB is communicated to the public to improve their understanding of the product. Acknowledgment forms are generally not included with the release of CIBs.
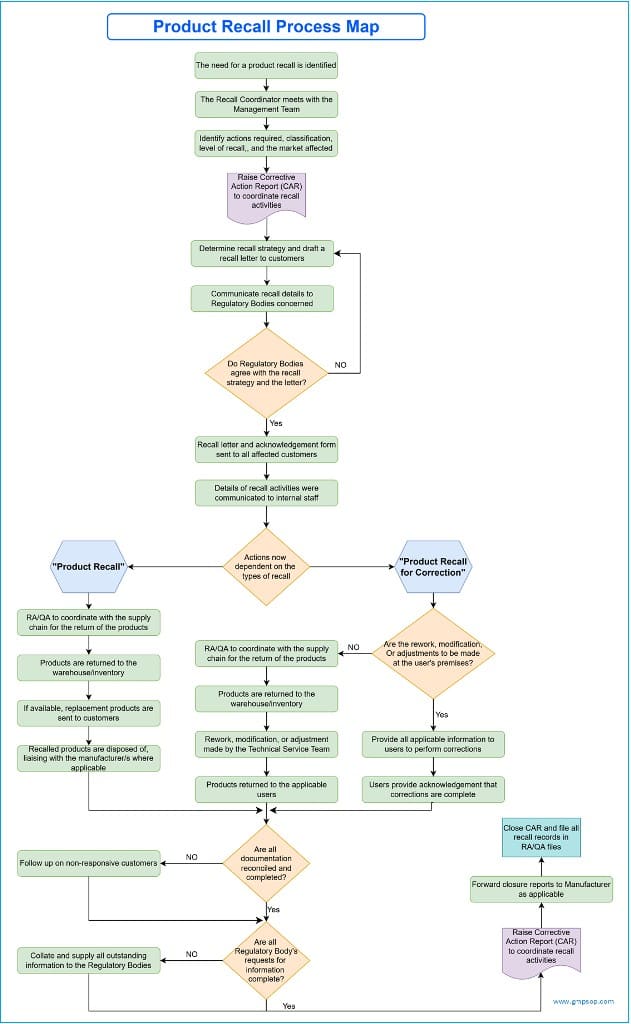
Initiating Pharmaceutical Product Recall
Regulatory and quality assurance teams typically coordinate a pharmaceutical product recall with oversight from senior management. It is a structured process with formal documentation and pre-defined roles, including the appointment of a recall coordinator.
The applicable regulatory body will also appoint a nominated recall coordinator as a point of contact for the company.
As applicable, senior representatives from regulatory affairs, quality assurance, logistics, sales and marketing, and customer service should establish a product recall team.
The product recall coordinator is responsible for inter-site liaison and ensuring that all details are communicated and appropriate approval for initiating a major product recall is obtained by the regulatory affairs and quality assurance. This approval must always be obtained in writing.
The product recall coordinator must also provide the regulatory affairs and quality assurance with the documented rationale for not conducting any recall, correction, or removal actions in cases where this has been considered but decided against.
In cases where a manufacturing site initiates the product recall, the appropriate manufacturer will formally notify the sponsor company of the reason, products affected, and actions to be taken.
Types of Product Recalls
i. Product withdrawal
The product type or model is permanently withdrawn from supply because it poses a known or suspected unacceptable risk to the safety and well-being of the public.
This recall may be voluntary, initiated by the product’s sponsor, or may result from a directive from a regulatory body.
ii. Batch (product) recall
A nominated amount of a product type or model is recalled from customers or the manufacturer’s stock due to a known quantity having a defect that is known to pose or suspected of posing an unacceptable risk to the safety and well-being of the public.
In this category, the known quantity is typically identified by its serial number range, manufacturing lot number, manufacturing date, or other means of distinguishing the affected products.
In these cases, only the affected products require recall.
This recall may be voluntary, initiated by the product’s sponsor, or may result from a directive from a regulatory body.
iii. Recall for product correction
This category applies where it has been identified that a product currently being supplied to the public could have modifications to the device itself or amendments made to any written information related to the device, such as operator’s manuals, instructions for use, and product labelling, and that such modifications may improve the safety and efficacy of the product.
The requirements for product correction are undertaken with close communication between the sponsor and the customer to ensure that the minimum amount of customer disruption is incurred while the product is modified.
Regulatory Bodies permit such product modifications to be undertaken progressively at the customer’s or the sponsor’s premises or a location agreed upon by both parties.
The sponsor may make this recall voluntary or follow a directive from a Regulatory Body.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
How to communicate product recall activities
Clear and timely communication with stakeholders is essential during recalls.
Effective and timely communication between stakeholders must be maintained, especially between the manufacturer and the regulatory body.
a. Communication with regulatory bodies
Each regulatory body has its own format for communicating the full details of the incident or product recall (e.g., a uniform recall procedure for therapeutic goods).
This document stipulates the actions to be undertaken, the documentation to be raised, and the time frames for the company to meet its regulatory reporting requirements on product recalls.
Some regulatory agencies have established contact lists for device sponsors to reference when issuing safety alerts or product recall notifications.
The list contains the names and email addresses of key hospital/health authority personnel, and sponsors must ensure that electronic copies of regulatory announcements are sent to these individuals via email and any postal communication.
b. Quality records
All documentation raised during processes related to adverse events and product recalls is to be considered a quality record and processed and archived in accordance with the quality procedure.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Pharmaceutical Product Recall Checklist
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit
Product recall activities in the pharmaceutical industry can be overwhelming, complex, and urgent. Many stakeholder communications and corrective actions require prompt and effective completion. The larger the business, the rigorous the recall process.
A product recall checklist is a good idea for keeping track of all activities. It serves as a visual aid to represent the progress of actions. When completed, the checklist can become a tool for reconciliations and proof of activity completion.
Below is a standard example of a recall checklist.
– Recall initiated/notified to Management?
– Corrective Action Report (CAR) raised?
– Impacted Markets: (list of countries affected).
– Recall Action: (Specify if only Recall or Recall for Product Correction).
– Classification of Recall: Specify class of drugs I, II, or III.
– Level: Select between Consumer, Hospital, or Inventory.
– Management Team Alerted?
– Media Release to be released? NOTE: Must be approved by the Regulatory Body’s Recall Coordinator.
– Management Team members to be involved?
– Any affected stock quarantined (Stop Supply)?
– Recall-Strategy agreed by Senior Management?
– Identified other CARs affected?
– Product Registration numbers identified?
– Product Distribution Records defined and recovered?
– Affected customers notified by phone to quarantine stock pending recall or possible recall (if applicable)?
– Was the manufacturer notified of recall actions and proposed strategy?
– Have regulatory bodies been notified of the recall and proposed strategy?
– Regulatory Bodies approve the recall strategy?
– Draft Recall letter submitted to the manufacturer (if applicable)?
– Draft Recall letter approved by manufacturer (if applicable)?
– Draft Recall letter submitted to Regulatory Bodies?
– Recall letter approved by Regulatory Bodies?
– Letter to Minister sent (if applicable)?
– Recall letter sent to affected customers & Customer Contact List checked?
– Customers are notified by: Specify the responsible person.
– Staff informed of recall and actions?
– Follow up on non-responsive customers?
– Reconciliation of Acknowledgement Forms?
– Are regulatory bodies’ requests for actions or information being addressed?
– Report to Regulatory Bodies 2 weeks after implementation
– Report to Regulatory Bodies 6 weeks after implementation
– Recall: Affected Stock and stock received from customers disposed of?
– If the stock is showing as ‘QA Hold’ on the system, is Logistics notified to remove it?
– Report closed by Regulatory Bodies?
– Have all actions been completed, and has the documentation been distributed and filed?
What is Market Action: Strategic Management Beyond Recalls
A market action in pharmaceuticals extends beyond product recalls and may involve:
– Market Withdrawal
– Field Correction
Market action policy shall be available at each manufacturing site that produces, stores, or distributes drug products, active pharmaceutical ingredients (API), or medical devices, and within each business unit responsible for any market action activities.
The market action procedure must be capable of being initiated promptly and at any time. The procedure shall include actions to address product recall, withdrawal, and/or field correction.
What triggers market actions
Market actions shall be considered in a number of situations, including, but not limited to, the following:
– Results of a product complaint investigation,
– Review of product retention samples of a distributed product lot,
– Material erroneously released to stock without quality team authorization,
– Mislabelled marketed product,
– Failure of a marketed product lot to meet specifications.
When a product market action is identified, a stop distribution notice (SON) shall be issued for all affected batches and placed under quarantine-hold status.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
Market Action Procedure
Each pharmaceutical business unit must have a market action procedure that meets global and local regulatory needs. When initiated, the Market Action Leader (MAL) oversees the implementation.
1. Market Action Governance and Initiation
A Market Action Coordination Committee (MACC) must be established for recalls conducted in the United States and its territories.
This committee must include representatives from the following functions:
– Market Action Leader
– Quality Operation
– Regulatory Affairs
– Distribution/Logistics
– Marketing
– Country General Counsel (Legal)
– Media Relations (if information releases are involved)
For market actions outside the US, the Market Action Leader for the respective country leads the coordination.
The regulatory affairs principal or business unit quality contact may assume this role, working with the quality team to implement necessary actions.
If contract manufacturers manufacture the imported products involved in market action, the quality team at the manufacturing site must be informed if the market action is initiated due to local regulatory team requirements.
All country regulatory affairs managers and qualified persons (QPs) must interact with regulatory authorities to ensure regulatory alignment and compliance.
2. Decision-Making and Authorization
The quality team at the manufacturing site is responsible for recommending and initiating a market action. This recommendation must be formally documented in meeting minutes.
– In the US, the MACC decides to initiate a market action.
– Outside the US, the decision is made by the country’s regulatory affairs principals or the business unit quality contact.
If the site quality operation (QO) leader decides to recall a batch (e.g., for personal liability concerns), Marketing cannot override this decision.
Should a regulatory team initiate a market action, all communications must occur through the regulatory affairs principals and/or the responsible site quality team or qualified person (QP).
3. Official Initiation and Notifications
The Market Action is officially initiated when a decision is made that a product recall, market withdrawal, or field correction is necessary.
i. In the US, this is communicated via QSTS principals through MACC meeting minutes or a formal memorandum.
ii. For international actions, the QORL, COQA Leader, or a QSTS representative must issue a global notification. It should inform:
– Quality Operations
– Regulatory Affairs (RA)
– Medical Affairs
– In Europe: Business Unit Quality Contacts and EU Pharmacovigilance QP
– Senior management: Marketing, Legal, Sales, Distribution/Logistics, Supply Chain Management
Each country’s RA principal or BU quality contact must assess the need for further notification to local authorities and document it per local regulations. If supply chain disruptions occur, the appropriate country authority must be informed.
4. Specific Market Action Strategy
The market action leader(s) must prepare a specific market action strategy for each market action. This strategy must include:
– Type of Market Action
– Depth of Market Action (e.g., wholesale, retail, patient)
– Health risk classification
– Notification method to customers or the public
– Extent of effectiveness checks (if required by regulation)
If required, the regulatory team must review and approve this strategy.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
5. Health Hazard Evaluation
The medical affairs and/or safety principals assess the seriousness of potential health hazards. Their evaluation must be shared with:
– Country Regulatory Affairs principals
– Impacted country regulatory authorities (for recall classification)
Assessment criteria include:
– Existing disease or injuries
– Contributing health conditions
– Likelihood and detectability of hazard
– Impact on vulnerable populations
– Hazard severity and reversibility
– Short- and long-term consequences
6. Inventory and Distribution Management
Accurate and detailed inventory and distribution records of APIs, medical devices, and drug product lots or batches must be readily accessible to support market actions.
Responsibility for providing this data lies with distribution and logistics services, depots, and supply points. Required information includes:
– Quantity in stock and distribution
– First and last distribution dates
– Names and addresses of customers (US only)
Product lots involved in a market action must be isolated and stored in designated areas. The site quality team holds final authority for disposition decisions.
7. Contract Manufacturer Involvement
If a contract manufacturer is involved, the primary liaison is the Director or Team Leader of contract operations, quality assurance, or the responsible site quality team. They serve as the contact between the market action leaders and the manufacturer.
8. Communication Management
8.1. Internal and Public Communications
When public releases are necessary, drafts must be reviewed and authorized by:
– General Counsel (Legal)
– Regulatory Affairs
– Business Unit Quality Contact
– Marketing
– Medical Affairs
– Media Relations
Site Global Research and Development principals must be informed if clinical studies may be affected.
8.2. Customer Communications
Customer notifications must:
– Be concise and direct
– Clearly identify the product and affected lot numbers
– Explain the reason for the Market Action and potential hazards
– Instruct customers to cease use or distribution immediately
– State if downstream customer notification is required
– Provide return instructions and response options (e.g., prepaid postcard)
9. Execution and Follow-up
The market action leader, supported by the site quality team, oversees:
– Identification of affected product/lot numbers and distribution timeline
– Drafting and coordination of Market Action communications
– Notification procedures
– Depth and reach of Market Action
– Maintenance of returned material records
– Execution of required Effectiveness Checks
– Preparation of all status and final reports
– Verification of returned product disposition
– Records must be maintained according to retention standards.
10. Effectiveness Checks
Effectiveness checks are conducted to verify that customers have received notifications and taken the necessary action.
They must be conducted when regulations require and may be coordinated by the market action leader or the site quality team.
11. Mock Market Actions
To ensure readiness, manufacturing site principals must conduct mock market actions regularly. These simulations assess the adequacy of procedures and training.
A mock action may be waived if an actual market action occurs within the designated timeframe.
For human health products marketed in the US and its territories, QSTS is responsible for conducting mock recalls, as needed.
250 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents are included each month. All written and updated by GMP experts. Check out sample previews. Access to exclusive content for an affordable fee.
12. Documentation and Reporting
12.1. Regulatory Documentation
Regulatory affairs or the responsible quality team must submit the following to regulatory authorities, when required:
– Market Action Notifications
– Status Reports
– Follow-up Reports
– Final Reports
12.2. Market Action Final Report
The final report, prepared by the market action leader, must include:
– Identification of drug product, medical device, or API
– Description of the Market Action
– Quantities distributed and returned
– Final product disposition
– Date the action was closed
– Approval signature
For US recalls, the report must also include:
– Number of customers contacted
– Number of responses received
Conclusion
In conclusion, this article provides a brief overview of managing patient incidents, adverse events, safety alerts, product recalls, and other market actions, including product withdrawals and field corrections.
During adverse event notification and product recall, it is important to ensure that all actions comply with regulatory requirements and are within the necessary timeframes by clearly defining the responsibilities of key individuals and the reporting and communication processes.
Use a risk-based approach to determine if a patient incident is categorized as an adverse event and if a product recall is necessary.
Please maintain effective and timely communication with the regulatory authority at all times.
FDA guidance on pharmaceutical product recall and ISO 13485 standards for medical devices provide frameworks for post-market surveillance and control mechanisms for managing adverse events and product recalls. This ensures that product recalls are handled efficiently and effectively, safeguarding public health.
By embedding these processes into your quality systems and practicing readiness through mock exercises, you can respond swiftly and effectively to any post-market event, safeguarding your products, brand reputation, and—most importantly—patients’ health.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.