How to effectively control packaging materials in pharmaceutical
- Published on: Dec 10, 2023
Pharmaceutical manufacturers implement numerous processes to control packaging materials to avoid costly mix-ups.
Particularly the printed packaging materials where product information is presented.
Printed packaging materials typically include product names, active ingredients, concentration, batch numbers, expiry dates, registration numbers, barcodes, etc. Commonly used packaging materials are cartons, inserts, leaflets, printed foil, etc.
Customers expect pharmaceutical products to be safe and effective until the expiry period.
One of the ways to ensure that medicinal products are safe and effective is by packing finished products into packaging materials imprinted with correct information.
Packaging and labeling mix-ups can potentially result in serious health consequences. As such, GMP rules require that products be correctly identifiable with a registered name, ingredients, strength, and batch information.
There must not be any unspecified components.
Accurate labeling of medicines is critical to patient health. Therefore, manufacturers pay particular attention to getting the right medication and strength into the right container with the correct labels and instructions for use.
Any error here could cost lives and certainly result in a recall.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Regulatory requirements for the control of packaging materials
According to US FDA CFR section 211.130, written procedures shall be designed to ensure correct labels, labeling and packaging materials are used for drug products.
You must follow the written procedures to control packaging materials all the time. These procedures shall incorporate the following features:
(a) Prevention of mix-ups and cross-contamination by physical or spatial separation from operations on other drug products.
(d) Examine packaging and labeling materials for suitability and correctness before packaging operations and document such examination in the batch production record.
(e) Inspection of the packaging and labeling facilities immediately before use to ensure that all drug products have been removed from previous operations. You’ll need to conduct inspections to ensure that packaging and labeling materials unsuitable for subsequent operations are removed. Could you document the results of the inspection in the batch production records?
Top reasons for drug recalls
Product recalls can cause enormous losses to the company, damage the brand integrity, and harm the relationships between manufacturer and consumer.
Following is a list of reasons medicinal products are often recalled. If you check carefully, you will find that some of the top reasons for drug recalls are packaging and labeling issues.
– Sub-potent (single-ingredient drugs)
– Impurities/degradation products
– Temperature abuse
– Lack of assurance of sterility
– Correctly labeled product in incorrect carton or package
– Failed dissolution test requirements
– Labelling illegible
– Label mix-up
– Chemical contamination
– Microbial contamination of non-sterile products
– Marketed without an approved NDA/ANDA
– Stability data does not support the expiration date
What are the potentials for mislabelling?
Packaging mix-ups, often called “mislabelling,” are among the most common causes of a medicinal product recall. Therefore, you must establish a proper mechanism for packaging material control to eliminate all types of mix-ups.
It would be best to implement special care in the packaging areas to ensure that batch numbers and expiry dates are printed correctly.
Labels, cartons, and leaflets are printed with correct product information. These packaging materials are assembled correctly in the packaging lines with the right product to fill every time.
When you have to rework a faulty batch, follow the approved protocol, complete line clearance, and document and record all packaging activities to ensure the control of packaging materials.
It is also a GMP rule that mislabelled products should never be “over-labeled.” Unless you are careful, it is very easy to make an error during rework, or the over-label may be dislodged later, causing the product to be mislabelled once again.
A product is “mislabelled” if the labeling and packaging information does not accurately reflect the contents of the container.
For example, if a label indicates that a bottle contains 100 capsules but less than 100 capsules inside, this is classified as a case of mislabelling.
A more extreme and potentially dangerous example is if the product was stated as “tamper-evident,” but there is no evidence of tamper-proof seals.
It will also be considered mislabelled if it does not conform with the marketing authorization and claims.
As part of product manufacture, companies must register the product’s labeling details with the regulators. If there is any deviation from these label specifications, the company is in breach of regulations.
Origins of tamper-evident packaging
In 1982, a deranged person in the US sabotaged Tylenol by injecting cyanide into capsules in supermarkets, which eventually killed six people. The FDA responded by making tamper-evident packaging a legal requirement for over-the-counter products, and the Federal Anti-Tampering Act passed in 1983 made it a crime to tamper with packaged consumer products.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
How to control packaging materials?
GMP rules are in place to control packaging materials and operations to prevent mix-ups in packaging. According to PIC/S, these control of packaging materials include:
– Control over the purchase, handling, storage, and segregation of printed packaging materials
– Line clearances and identification
– Packaging verification
– Ensuring the containers are clean before filling
– Labelling immediately after filling
– Checking and recording the printing operation
– On and offline label printing controls
– Using labelling that is resistant to fading or erasing
– Investigation of reconciliation discrepancies
– Destruction or return of unused material
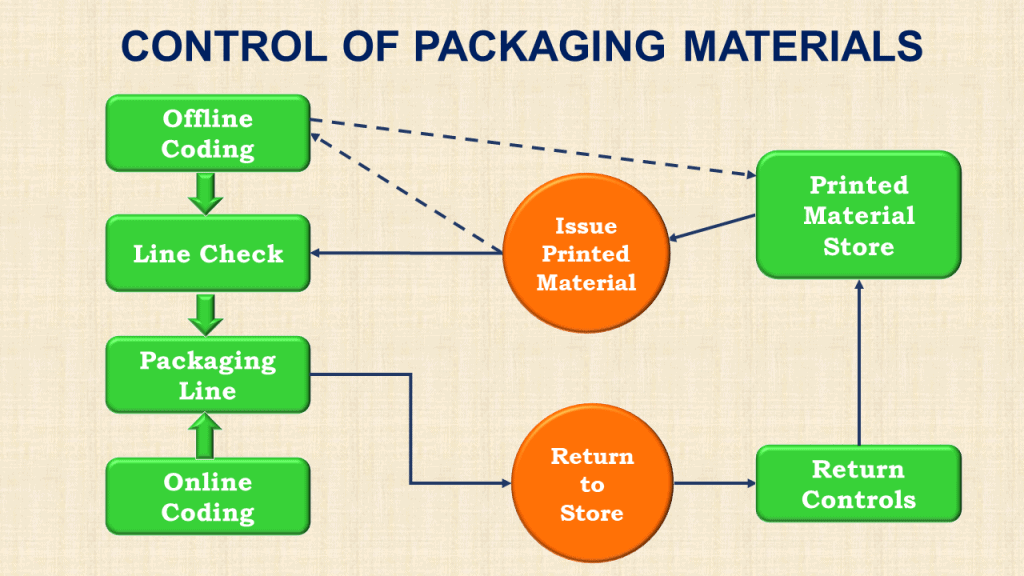
1. Controlling printed packaging materials
Many mislabelling incidents commence with the wrong issue of labels or other printed material from the store.
The GMP rules clearly state that each issue of printed material from the store must be accompanied by requisition paperwork and that there is a step of cross-checking that labels match the documented request before issue.
Issuing of correct printed material is the first point of control. It is much better to prevent incorrect labels from being issued in the first place rather than finding them (or not) later on.
Mislabelling can cause incorrect consumption of medication, which can result in severe injury or even death of patients.
There are GMP controls over how manufacturers handle printed material. Printed material should be verified, kept separate, and always secured.
This ensures the distributed product does not result in recall or withdrawal from the marketplace. These measures help to protect patients from taking the wrong medicines.
The term “printed packaging material” includes:
– Labels for containers (rolls of labels and cut labels)
– Package leaflets (inserts and outserts)
– Labels for cartons or packaging
– Printed containers (e.g., lubes)
2. Control of packaging materials at the printers
Pharmaceutical labeling suppliers are a significant component of a successful packaging and labeling process. Their adherence to change control and GMP compliance should be as strong as the manufacturer’s.
You should conduct routine audits of the printed material suppliers to ensure they maintain GMP standards.
The audit should focus on the printer’s line clearance practices, counting accuracy, and an acceptable system for handling and disposing of waste.
Printers should follow written and GMP-approved operating procedures to tighten packaging and labeling control. Tracking materials and keeping good records are needed to help prevent mix-ups.
Where different batches of printed material are being produced simultaneously, you should physically separate them from one another to avoid mix-ups.
Other in-process controls at the printers include:
– Using identification numbers that are unique to each revision
– Optical bar codes for verification
– Artwork control
A common occurrence in the printing industry is the use of “make ready” packing, which refers to the first few sheets or labels that pass through a printing machine before the actual run starts.
There is a natural desire to save on material waste by minimizing make-ready or using it to set up other processes, but the wiser course of action is to discard it at each step in the run.
The cost of an improper matching of packaging materials can overpower the potential savings of reducing waste.
3. Control of packaging materials at the warehouse
Receiving printed material at the inward goods bay is crucial for producing a quality product.
Please make sure the correct printed materials are received, which will be released later to the packaging area.
When the printed material arrives from the printers, you should apply a unique identifying number and place it into quarantine.
Following receipt and quarantine, quality control (QC) examines and verifies printed materials against standard specimens and specifications to prevent any mix-ups or errors.
QC must take particular care with sampling and testing because many labels, cartons, and leaflets look alike. Packaging and labeling samples should not be returned to inventory.
Printed materials can be released for use by quality assurance only after inspection and approval are done.
a. Arrival of printed materials
– You should count roll labels either on receipt or at issue.
– Supplier counts are only acceptable if the supplier is specifically qualified and the supplier certifies the exact count for each roll.
– Supplier numbering of labels is an acceptable alternative.
– Ensure Cut labels are counted and effectively verified by the manufacturer because of the risk of mix-up.
b. Quarantine of printed materials
– Printed material must be quarantined from use until it has been thoroughly quality-controlled.
– Quarantining involves placing the received material into a locked, separate store secured from unauthorized access.
– When they handle printed material, all personnel should double-check that the “Quarantine” label has been replaced by an “Approved for use” label. If it hasn’t, do not use the printed material.
4. Quality control of printed materials
QC uses sampling plans to specify the number of samples of printed material to be taken. There are several types of sampling plans QC uses, e.g., statistical, targeted, and zero acceptance numbers.
Although QC uses all of them for printed material in some respects, the reasons why each is chosen are beyond the scope of this article.
Suffice it to say sampling plans for printed material account for at least the following:
– The quantity and quality of printed material received
– The quality of printed material required
– The nature of the material (e.g., primary and printed materials)
– The production methods
– An audit of the printed material supplier
5. Printed material issue, coding, and returns
Labels and cartons (outers) can be coded with batch numbers and expiry dates offline or online.
Offline coding requires a specific packaging record and all the usual checks and clearances.
Online coding is usually done when the printed material is applied to the primary packaging.
Occasionally, unused, uncoded printed material is returned to the store for later use. The return process requires specific checks of its own.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
6. Control of packaging materials storage and coding
a. Offline coding
Some manufacturers code the batch number and expiration dates “offline.” i.e., in a separate production step called printed mailer coding.
When offline coding is used, you should conduct line clearances and double-check that the packaging line is used for the same batch and expiration.
Correctly coded printed packaging material may be returned to the printed material store for reissue or transferred directly to the packaging line.
b. Line check
Before packaging begins, you should conduct a line clearance to ensure no potential for mislabelling.
The line clearance involves at least two things:
– Complete removal of all previous product documentation and printed material of the prior batch.
– Double-check that the correct printed material has been selected and presented to the line when new printed material is added to the line. This check must be recorded and authorized in the packaging record.
c. Packaging line
During the actual packaging, many things may need to be corrected. So, you will need to conduct frequent online checks.
– When printed packaging material is first presented to the line, please ensure that only the correct material has been introduced.
– When additional packaging materials are brought to the line, double-check them for correctness.
– Double-check the items on startup when the line stops for any reason.
– When automatic online verifiers are used, verify that they read correctly before use.
d. Online coding
Some companies prefer to print the batch number and expiry dates directly “online,” i.e., apply the information. In contrast, the printed material is assembled with the product to minimize the chance of mix-ups before labeling.
This approach requires additional controls in place.
It is essential to double-check the setup for online coding to verify that only the correct batch number and expiry date are programmed in.
As the packaging run progresses, it is essential also to check that the inking or printing has stayed the same, which could leave units incompletely coded or not coded at all.
e. Issue of packaging materials
Printed material is to be checked against the work order to verify the following:
– The assigned product and lot number
– The printed material code and version number
– The batch number and expiry date (if the printed material is already coded)
f. Return control of packaging materials
Printed materials returned to the store should only be handled by authorized personnel. The person returning the printed packaging material should never directly put it back into storage.
Extreme care must be taken when returning the printed material to storage because it is well-known that mix-ups occur if returned items are placed in the wrong storage locations.
The alternative to returning unused printed material to the store is outright destruction.
If the coded printed material isn’t used on the line, it must be destroyed to avoid ending up in subsequent batches.
When the printed material is destroyed, it must be done securely, accompanied by a signed record of destruction.
Some organizations will not return printed material to the store because of the potential to mix up the returned item.
It is a GMP regulation that coded printed material is never returned to the store for reuse. The reasons for this should be apparent, as coding is always unique to a batch.
g. Line clearance & setup
In a busy packing hall, many products are generally processed at once. Unless specific GMP controls are in place, a rogue item of printed material will inevitably end up on a different product.
This may not get detected later and could injure patients and cause a recall. Proper line segregation helps to physically separate different printed materials, much of which look similar.
A combination of physical segregation, double line checks when new printed material arrives, and detailed line clearances are critical GMP labeling controls.
Line clearances are used in the labeling and packaging area as another control to prevent product mix-up.
The following steps must be conducted and verified before the start of the packaging and labeling operation:
– Clean the area and machines,
– Remove all previous products and machines from the area.
– Remove all waste from the area and machines.
– Reconcile all printed material and products from the previous batch.
Once the above steps are complete, please make sure to document all activities that the area and machines are inspected and found to be clear of all previous products.
Before starting any filling and labeling operation, please look over the line thoroughly following a standard operating procedure to make sure that all materials, products, labels, and records from previous operations have been removed.
The person responsible should initial the batch record to show that this check has been carried out.
Label the line clearly to show the product (and the strength of the product) to be packaged.
The label counters or code readers should be tested to verify that they work correctly.
Carefully check all labeling and packaging material to match the descriptions in the batch packaging records.
7. Online control of packaging materials
Using reliable controls such as bar code readers is an expected practice in packaging operations. Removal or turning off a barcode reader takes away a vital packaging control.
At the same time, regular misreading is also not acceptable. The reader should be repaired, returned to the line, and re-validated to avoid suspected false reading.
During the packaging and labeling operation, GMP rules specify the number of necessary online (in-process) controls.
Online controls of packaging materials should include the following checks:
– The bulk material and printed material
– The appearance of the product (e.g., chipped labels, particles in sterile injectable)
– The equipment used (including the label verifier)
– The count or measure of the filled container
– The label appearance and adhesion coding
– The tightness of the cap
– The seal integrity of the packs
When these checks are done by production staff, the check should be audited by the Quality Department.
8. Reconciliation of packaging materials
Reconciliation of printed materials is one of the critical GMP packaging material controls. The reconciliations indicate how many printed material items need to be accounted for.
It is well-known that incorrect calculations are common mistakes in this area, for example, assuming that any unaccounted-for numbers must be rejected. While reconciliation limits should be tight, around 100%, every result isn’t expected to be exactly that number.
240 SOPs, 197 GMP Manuals, 64 Templates, 30 Training modules, 167 Forms. Additional documents included each month. All written and updated by GMP experts. Checkout sample previews. Access to exclusive content for an affordable fee.
Conclusion
Pharmaceutical manufacturers rigorously control packaging materials, focusing on printed details like product names and expiry dates. Accurate packaging prevents mix-ups and complies with Good Manufacturing Practice (GMP) rules.
The top reasons for drug recalls often involve packaging and labelling issues, emphasising the need for robust controls.
Mislabelling is a common cause of mix-ups and product recalls, highlighting the importance of effective packaging material control.
GMP rules cover packaging materials’ purchase, handling, and storage, emphasising line clearances, verification, and strict controls during labelling and storage.
Tamper-evident packaging originated from a 1982 incident, with GMP rules specifying controls at printers and warehouses.
In summary, control of packaging materials in the pharmaceutical industry is governed by GMP rules, ensuring accuracy, preventing mix-ups, and safeguarding patient health.

Author: Kazi Hasan
Kazi is a seasoned pharmaceutical industry professional with over 20 years of experience specializing in production operations, quality management, and process validation.
Kazi has worked with several global pharmaceutical companies to streamline production processes, ensure product quality, and validate operations complying with international regulatory standards and best practices.
Kazi holds several pharmaceutical industry certifications including post-graduate degrees in Engineering Management and Business Administration.