Guidance Summary 021 - 030
Guidance 021 Summary - Establishing and Extending Clean Equipment Hold Times
This guidance describes considerations and risks for determining if the establishment of clean equipment hold times (CEHT) for equipment producing drug product and Active Pharmaceutical Ingredients (API). This guidance does not apply to clean equipment after sterilization.
Grouping of equipment to establish CEHT is also explained.
The clean equipment hold time is defined as the time between the last step of the cleaning procedure.
The risk of contamination of clean equipment with dust, etc due to exposure to the environment. For this risk, it is required that equipment either be held clean in a controlled clean environment or protected from the environment.
The following factors should be considered to determine the level of risk for clean equipment hold times.
Considerations for the risk of contamination of clean equipment with non-viable particulates (e.g. dust) due to exposure to the environment.
Conditions of equipment storage, such as protection from an uncontrolled unclean environment. Protecting equipment such as with plastic sheaths or bags, closing lids, storing in clean areas, etc. are methods of minimizing negative effects of storage with respect to potential non-viable particulate contamination.
If CEHT must be monitored (i.e. tracked) and/or validated, establishing or extending the clean equipment hold time for a group of equipment may be done by obtaining data for one representative item in the group.
For example, data generated for 1 of 5 coating pans, 1 of 4 tablet presses, or 1 of 4 centrifuges would apply to the other pieces in the group, if the pieces are documented to be equivalent with respect to cleanability.
Sampling locations may be designated as most likely to contain contamination upon storage. Locations may or may not necessarily be the same locations as those most likely to contain residual product residue.
A rationale for sampling locations for CEHT is suggested. If an established CEHT is exceeded the equipment should be cleaned or rinsed prior to next use. Additional sampling after the extended CEHT can also be considered.
Guidance 022 Summary - Evaluating Non-Cleaned Equipment Hold Times for Cleaning Validation
This guidance outlines considerations and risks associated with hold times between equipment use and cleaning.
The non-cleaned equipment hold time period is defined from the “end of manufacturing” to the start of cleaning. The beginning of cleaning is defined when a cleaning activity is initiated on the equipment. Maximum allowable time intervals for periods between API equipment use and cleaning (non-cleaned or “dirty” hold time) are required to be specified unless there is an approved documented rationale or data demonstrating the time interval is non-critical. Non-cleaned equipment hold times for APIs are not required to be validated.
Consideration of the hold time of equipment after manufacturing use and before cleaning is important because it may impact the equipment cleaning.
Drying of product on the surface.
- Certain organic compounds, APIs, waxes, or polymeric formulations may harden on drying or standing, making it more difficult to remove. Example is polymethylacrylates as coating polymers.
- In some cases, it is possible that after drying of the residue during normal manufacturing, further increase in hold time will have no effect on the difficulty of cleaning to remove product residue. For example, this may be the case when processing conditions are significantly more severe than idle hold time conditions (e.g. drying a product in a Rosenmund Filter for 3 days at 70 degrees C versus idle hold time of the empty non-cleaned filter at room temperature).
Lab recovery study data for the product residues may have been generated when residues are dried on representative sample surfaces (coupons). These data may also support non-cleaned equipment hold time rationales or data demonstrating that they are not critical. This may be more applicable for APIs, where only a single component is typically removed during cleaning (versus active and excipient mixtures in Drug Product).
Many GMP sites currently requires one validation run for drug product equipment to validate hold times between equipment use and cleaning. The rationale for one run of data is that this is considered current industry standard based on recent external benchmarking data. Reproducibility will have been demonstrated in the replicates of the validation study. Some GMP sites have validated with 3 runs to assess variability. Other sites have validated with 1 run.
Guidance 023 Summary - Evaluation of Changes for Potential Impact on Process Validation
This guidance applies to validated processes and identifies examples of changes for which examining the validation impact of a change should be considered. This evaluation should include assessment of the validation impact of the change. Major changes require validation, while documented evaluation of minor changes are typically documented using the site change management system.
Evaluating proposed changes to a process shall include a documented assessment of the validation impact of proposed changes. Assessment of the validation impact of minor changes is typically documented using the site’s change management work process.
Every proposed change should be assessed to determine the potential impact of the change and to consider the potential impact to product quality from the adopted change.
Evaluation of the quality impact of the change should take place as close as practical to the step in the process where the change was made. The system owner or Technical Services may typically propose the change and should participate in assessing the impact of the change. The Quality organization must be included in approval of the assessment of impact of the change to a validated process.
Types of Major Changes and Points to Consider with this Change
| Type of Major Change | Points to consider with this change |
|---|---|
| Change in critical unit operations (e.g., addition, deletion, change in order of steps, repetition of an existing unit operation on a routine basis); | Validation of part or all of process is recommended. Process validation is needed if a change in the process is expected to have measurable impact on product quality or process performance, as determined by risk assessment. Is the change supported by data from a development lab? Is the process still capable of providing good quality product if a material specification is relaxed? Tightening of a specification may not require validation as this will typically not challenge the capability of the process. Is stability of API or DP affected? See also example 1 in the text. |
| Change of source or specification of a critical material (e.g., regulatory intermediate or API starting material); | |
| Modified operating conditions (e.g.time, temperature, pH, reagent stoichiometry) that impact CQAs; | |
| Change that could impact acceptable microbiological quality of the product. |
Guidance 026 Summary - In-Process and Bulk Drug Product Holding Times
This Guidance sets out guidelines for the determination and validation of in-process and bulk product holding times.
When appropriate, time limits for the completion of each phase of production shall be established to assure the quality of the drug product.” .This regulation could be interpreted to include the time for holding bulk product as part of the production process. “holding times (includes storage times) studies may be conducted during development or carried out in conjunction with process validation lots and shall be representative of full scale holding conditions.
For current marketed products, a historical review of product lot release and stability data may be used to substantiate hold times if hold times were not established as part of validation. The longest hold time used for the lots reviewed will become the validated hold time.
The product bracketing or matrixing approach may be used to group products with same/similar formulations or combination strength products. For multiple strengths of the same formula, use of lots with the highest and lowest dosage only may be justified for the study. Reasons for excluding a product from a bulk holding study should be justified. Typically, one lot is recommended for bulk holding studies.
Typically, if these in-process products are used within 24 hours of manufacturing, no bulk holding time studies are deemed necessary. An in-process product that is held for longer than 24 hours should be monitored for physical characteristics and microbial contamination. A solution/suspension should be held for the defined hold period. At the test points, a sample should betaken from the storage container and tested. Results obtained should be compared with the initial baseline data of the solution/suspension control sample results.
Typical tests include the following: Microbial count; Yeast/Mould count; Specific Gravity; and Viscosity.
Guidance 027 Summary - Demonstration of Active Pharmaceutical Ingredient Batch Homogeneity
This procedure provides guidance for performing a homogeneity evaluation in support of API process validation.
- Materials to be tested
- Selection of test methods for examining homogeneity
- Sampling plan – when to collect samples, from what locations, and the of samples
- Selecting acceptance criteria for evaluating homogeneity test results.
Homogeneity is the acceptable distribution of chemical and physical properties within a batch, based on predefined criteria. The intent of examining homogeneity during the validation is to demonstrate that the quality of a sample collected from any location within a batch is representative of the quality of the entire batch.
For large molecules the evaluation of homogeneity must consider the consistency of the profile of heterogeneity of product-related molecular variants. This profile should be consistent throughout a batch and similar between batches.
Three measurements are typically considered for a given homogeneity study: one to demonstrate chemical homogeneity, one to demonstrate physical homogeneity (if appropriate), and one to demonstrate the effectiveness of the drying process (if appropriate). Appropriately chosen analytical tests in these categories usually eliminate the need to perform other analytical tests to show homogeneity.
Sampling should target potential in homogeneity. An API process generally prepares a material that is a single substance rather than a mixture of materials (such as that found in a drug product), and a well-mixed API batch is typically obtained near the end of processing. Operations that potentially introduce inhomogeneity in APIs are readily identified (examples include collection of caked material from a crystallization tank, non-agitated washing of filter cakes, and non-agitated drying of filter cakes), so sampling and analytical testing may be targeted at investigating suspected potential for inhomogeneity.
For an API quality attribute that is considered acceptable when it conforms to a limit or range (as is true for many physical properties), homogeneity may be established by demonstration that each sample of the API lot conforms to the limit(s) for that property. Analytical test results that are normally reported as “Meets Test” or “Acceptable” for chemical properties are not suitable for evaluation by statistical methods.
Guidance 028 Summary - Documentation Example for Continuous Quality Verification
Continuous Quality Verification (CQV) is an alternative approach to process validation. Documentation for a process may include some or all of the content described here, depending on the nature of the process. Other documentation may also be needed to support a CQV approach.
The process: A granulated drug product mixture is dried using a fluid bed dryer. Air is passed through the mixture to remove moisture, providing a mixture for compression into tablets.
Process Understanding documentation for this process includes a brief description of a process to dry a granulated drug product mixture, such as that described below.
The product is dried with a fluid bed dryer, which uses a through-the-bed flow pattern with air passing through a distribution plate and into the drying chamber, where it lifts the granular product and maintains the granules in a fluidized state. This bed of granulate particles displays fluid-like properties like that of a liquid. This fluidization provides intimate contact between particles and the warm (about 40 oC) air stream, providing an efficient means of transferring moisture away from the product particles.
While air flow, and temperature are considered potential critical process parameters (CPPs) for this operation, monitoring with NIR provides superior control and permits relaxation of time and temperature limits. Air flow and drying temperature must still be controlled within reasonable limits to reduce process variability in terms of the time needed for the drying operation. However, continuous monitoring with NIR, while not itself an operating parameter, is identified as the critical process control necessary to insure consistently meeting the product’s moisture CQA.
A plan should be established for the analysis of process trends. This plan should include a recommendation for the appropriate frequency for evaluation of process trends and what data should be reviewed.
Guidance 029 Summary - Documentation to Support Continuous Quality Verification
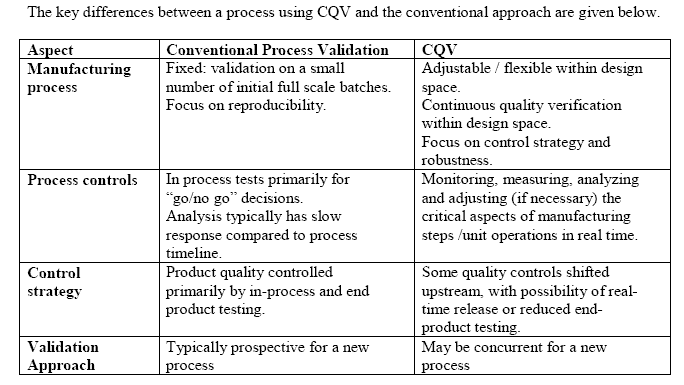
Continuous Quality Verification (CQV) is an alternative approach to process validation. One of the primary differences for CQV compared to a conventional discrete, 3-batch process validation approach is that the process is continually monitored, evaluated, and adjusted (when necessary) to achieve defined Critical Quality Attributes (CQAs) using validated in-process measurements, tests, controls, and process end points.
The CQV approach to process validation may be applied to both new and legacy processes when the necessary information is available. It requires a good understanding of the process and a process control strategy that ensures repeatable and robust performance of the process.
- Process Understanding:
Process design documentation is a pre-requisite for CQV and should include the following:
- a) Documented summary of scientific understanding of Product and Process(es)
During process development, the proposed process design is investigated and characterized by experiments, process modelling, etc. Critical Quality Attributes (CQAs) for the product must be defined and documented. Potential Critical Process Parameters (CPPs) to be controlled and monitored in order to achieve CQAs must also be identified whether using a conventional or a CQV approach
Differences between processes implementing CQV and those following a conventional approach:
Guidance 030 Summary - Guidance on Selection Criteria of Dose and Toxicity Data
For consistency, use of Acute Oral LD50 values obtained using rats as the study population is recommended to be used. The justification for utilising rat acute oral LD50 values is based on a commonly referenced article on this subject. Layton et al suggests that a safety factor to be used in calculating the Acceptable Daily Intake (ADI) be in the range of 1×10-3 to 5 x10-6. This factor is based on small mammal and oral rat data. The MAR formula, therefore, require the overall safety factor of 5 x 10-6 {5 x 10-4 in the No Observable Effect Level (NOEL) calculation and another 1 x 10-2 in the
ADI calculation, which incorporates the NOEL}. The ADI is used in the Toxicity Maximum Allowable Residue (MAR) calculation. The safety factor of 5 x10-4 has been reported in other literature articles for NOEL and appears to generally be accepted in the industry.
The following points can be considered when selecting Dose data to be used in the calculations of Maximum Allowable Residue for Therapeutics (MART) or Dose MAR:
- In cases where multiple formulations of an API are being produced in Drug Product (DP) manufacturing, or if the specific dosage form and/or dose to be used is unknown (e.g. APIs for external sales), then the most conservative published minimum therapeutic dose (TA) of the API for all DP formulation uses should be utilised in the MART calculations.
- In the DP manufacturing plant where more than one dosage form or delivery system (e.g. oral tablet, injectable liquid, topical cream/ointment) of the same API are produced on a given equipment item, the minimum therapeutic dose [TA] (used in the numerator of the equation) is specific to the drug product dosage form/delivery system.
Site A produces a 300 mg and 400 mg oral capsule and at site B the 100 mg oral capsule is produced; published minimum therapeutic dose is 100 mg. Thus site A producing the 300 mg and 400 mg capsule would use 100 mg as the minimum therapeutic dose for the product. Published minimum therapeutic doses of an API usually reflect the pharmacological activity for a specific route of administration such as injectable, oral or topical.
